Abstract
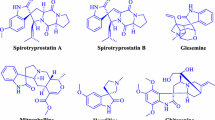
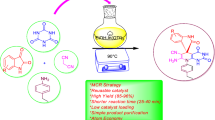
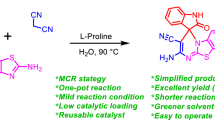
An efficient and environmentally sustainable synthetic protocol has been presented to synthesize structurally diverse spiroxindoles spiroannulated with indenopyrroloimidazoles, pyranopyrroloimidazoles, chromenopyrroloimidazoles, and imidazopyrrolopyrimidines involving three-component reaction of isatins, hydantoin, and β-diketones in the presence of green and sustainable bio-organic catalyst, β-amino acid, 2-aminoethanesulfonic acid (taurine), in aqueous media. The synthetic efficiency, operational simplicity, and reusability of catalyst make the present synthetic protocol cost effective, time efficient, and eco-friendly to synthesize molecules with structural diversity and molecular complexity and expected to contribute significantly not only to drug discovery research but also to pharmaceutical and medicinal chemistry.









Similar content being viewed by others
References
Poomathi N, Mayakrishnan S, Muralidharan D, Srinivasan R, Perumal PT (2015) Reaction of isatins with 6-amino uracils and isoxazoles: Isatin ring-opening vs. annulations and regioselective synthesis of isoxazole fused quinoline scaffolds in water. Green Chem 17:3362–3372. https://doi.org/10.1039/C5GC00006H
Simon M-O, Li C-J (2012) Green chemistry oriented organic synthesis in water. Chem Soc Rev 41:1415–1427. https://doi.org/10.1039/C1CS15222J
Dömling A, Wang W, Wang K (2012) Chemistry and biology of multicomponent reactions. Chem Rev 112:3083–3135. https://doi.org/10.1021/cr100233r
de Graaff C, Ruijter E, Orru RVA (2012) Recent developments in asymmetric multicomponent reactions. Chem Soc Rev 41:3969–4009. https://doi.org/10.1039/C2CS15361K
Brauch S, van Berkel SS, Westermann B (2013) Higher-order multicomponent reactions: beyond four reactants. Chem Soc Rev 42:4948–4962. https://doi.org/10.1039/C3CS35505E
Castro-Puyana M, Marina ML, Plaza M (2017) Water as green extraction solvent: principles and reasons for its use. Curr Opin Green Sustain Chem 5:31–36. https://doi.org/10.1016/j.cogsc.2017.03.009
Pirrung MC, Sarma KD (2004) Multicomponent reactions are accelerated in water. J Am Chem Soc 126:444–445. https://doi.org/10.1021/ja038583a
Chanda A, Fokin VV (2009) Organic synthesis “On Water”. Chem Rev 109:725–748. https://doi.org/10.1021/cr800448q
Rajarathinam B, Kumaravel K, Vasuki G (2017) “In Water”: organocatalyzed diastereoselective multicomponent reactions toward 2-azapyrrolizidine alkaloid scaffolds. ACS Comb Sci 19:455–463. https://doi.org/10.1021/acscombsci.7b00038
Cornils B (1995) Exciting results from the field of homogeneous two-phase catalysis. Angew Chem Int Edit 34:1575–1577. https://doi.org/10.1002/anie.199515751
Li C-J (2005) Organic reactions in aqueous media with a focus on carbon − carbon bond formations: a decade update. Chem Rev 105:3095–3166. https://doi.org/10.1021/cr030009u
Dallinger D, Kappe CO (2007) Microwave-assisted synthesis in water as solvent. Chem Rev 107:2563–2591. https://doi.org/10.1021/cr0509410
Ishikawa S, Hamada T, Manabe K, Kobayashi S (2004) Catalytic asymmetric hydroxymethylation of silicon enolates using an aqueous solution of formaldehyde with a chiral scandium complex. J Am Chem Soc 126:12236–12237. https://doi.org/10.1021/ja047896i
Shirakawa S, Ota K, Terao SJ, Maruoka K (2012) The direct catalytic asymmetric aldol reaction of α-substituted nitroacetates with aqueous formaldehyde under base-free neutral phase-transfer conditions. Org Biomol Chem 10:5753–5755. https://doi.org/10.1039/C2OB07193B
Wnorowski A, Yaylayan VA (2000) Influence of pyrolytic and aqueous-phase reactions on the mechanism of formation of maillard products. J Agr Food Chem 48:3549–3554. https://doi.org/10.1021/jf9913099
Wang Y, Rivera Vera CI, Lin Q (2007) Convenient synthesis of highly functionalized pyrazolines via mild, photoactivated 1,3-dipolar cycloaddition. Org Lett 9:4155–4158. https://doi.org/10.1021/ol7017328
Cho CS, Kim JH, Shim SC (2000) Ruthenium-catalyzed synthesis of indoles from anilines and trialkanolammonium chlorides in an aqueous medium. Tetrahedron Lett 41:1811–1814. https://doi.org/10.1016/S0040-4039(00)00035-6
Khadilkar BM, Gaikar VG, Chitnavis AA (1995) Aqueous hydrotrope solution as a safer medium for microwave enhanced Hantzsch dihydropyridine ester synthesis. Tetrahedron Lett 36:8083–8086. https://doi.org/10.1016/0040-4039(95)01680-G
Bose DS, Fatima L, Mereyala HB (2003) Green chemistry approaches to the synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones by a three-component coupling of one-pot condensation reaction: comparison of ethanol, water, and solvent-free conditions. J Org Chem 68:587–590. https://doi.org/10.1021/jo0205199
Totlani VM, Peterson DG (2005) Reactivity of epicatechin in aqueous glycine and glucose maillard reaction models: quenching of C2, C3, and C4 sugar fragments. J Agr Food Chem 53:4130–4135. https://doi.org/10.1021/jf050044x
Wang X-S, Zhang M-M, Zeng Z-S, Shi D-Q, Tu S-J, Wei X-Y, Zong Z-M (2005) A simple and clean procedure for the synthesis of polyhydroacridine and quinoline derivatives: reaction of Schiff base with 1,3-dicarbonyl compounds in aqueous medium. Tetrahedron Lett 46:7169–7173. https://doi.org/10.1016/j.tetlet.2005.08.091
Dwars T, Paetzold E, Oehme G (2005) Reactions in micellar systems. Angew Chem Int Ed 44:7174–7199. https://doi.org/10.1002/anie.200501365
Paprocki D, Madej A, Koszelewski D, Brodzka A, Ostaszewski R (2018) Multicomponent reactions accelerated by aqueous micelles. Front Chem. https://doi.org/10.3389/fchem.2018.00502
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163. https://doi.org/10.1152/physrev.1992.72.1.101
Salze GP, Davis DA (2015) Taurine: a critical nutrient for future fish feeds. Aquaculture 437:215–229. https://doi.org/10.1016/j.aquaculture.2014.12.006
Hofmann A (1963) the function of bile salts in fat absorption. The solvent properties of dilute micellar solutions of conjugated bile salts. Biochem J 89:57–68. https://doi.org/10.1042/bj0890057
Daneshvar N, Shirini F, Langarudi MSN, Karimi-Chayjani R (2018) Taurine as a green bio-organic catalyst for the preparation of bio-active barbituric and thiobarbituric acid derivatives in water media. Bioorg Chem 77:68–73. https://doi.org/10.1016/j.bioorg.2017.12.021
Shirini F, Daneshvar N (2016) Introduction of taurine (2-aminoethanesulfonic acid) as a green bio-organic catalyst for the promotion of organic reactions under green conditions. RSC Adv 6:110190–110205. https://doi.org/10.1039/C6RA15432H
Kong W-J, Chen X, Wang M, Dai H-X, Yu J-Q (2018) Rapid syntheses of Heteroaryl-Substituted Imidazo[1,5-a]indole and Pyrrolo[1,2-c]imidazole via Aerobic C2–H functionalizations. Org Lett 20:284–287. https://doi.org/10.1021/acs.orglett.7b03596
Mal K, Naskar B, Mondal A, Goswami S, Prodhan C, Chaudhuri K, Mukhopadhyay C (2018) Dihydroindeno[1,2-b]pyrroles: new Al3+ selective off–on chemosensors for bio-imaging in living HepG2 cells. Org Biomol Chem 16:5920–5931. https://doi.org/10.1039/C8OB01411F
Jeyachandran V, Muthu M, Kumar RR (2015) Efficient green protocol for the synthesis of novel Dihydroindeno[1,2-b]pyrroles. Synthetic Commun 45:1137–1144. https://doi.org/10.1080/00397911.2015.1005631
De AU, Saha BP (1973) Hydrindene derivatives as potential oral hypoglycemic agents: N-alkyl 1,2,3,3a,4,8b-hexahydroindeno[1,2-b]pyrroles. J Pharm Sci 62:1363–1364. https://doi.org/10.1002/jps.2600620834
Hanessian S, Papeo G, Angiolini M, Fettis K, Beretta M, Munro A (2003) Synthesis of functionally diverse and conformationally constrained polycyclic analogues of proline and prolinol. J Org Chem 68:7204–7218. https://doi.org/10.1021/jo0301447
Lehuédé J, Fauconneau B, Barrier L, Ourakow M, Piriou A, Vierfond J-M (1999) Synthesis and antioxidant activity of new tetraarylpyrroles. Eur J Med Chem 34:991–996. https://doi.org/10.1016/S0223-5234(99)00111-7
Fujimoto RA, Boxer J, Jackson RH, Simke JP, Neale RF, Snowhill EW, Barbaz BJ, Williams M, Sills MA (1989) Synthesis, opioid receptor binding profile, and antinociceptive activity of 1-azaspiro[4.5]decan-10-yl amides. J Med Chem 32:1259–1265. https://doi.org/10.1021/jm00126a019
Del Poeta M, Schell WA, Dykstra CC, Jones S, Tidwell RR, Czarny A, Bajic M, Bajic M, Kumar A, Boykin D, Perfect JR (1998) Structure-in vitro activity relationships of pentamidine analogues and dication-substituted bis-benzimidazoles as new antifungal agents. Antimicrob Agents Ch 42:2495–2502. https://doi.org/10.1128/aac.42.10.2495
Von der Saal W, Hoelck JP, Kampe W, Mertens A, Mueller-Beckmann B (1989) Nonsteroidal cardiotonics. 2. The inotropic activity of linear, tricyclic 5-6-5 fused heterocycles. J Med Chem 32:1481–1491. https://doi.org/10.1021/jm00127a015
Toja E, Selva D, Schiatti P (1984) 3-Alkyl-2-aryl-3H-naphth[1,2-d]imidazoles, a novel class of nonacidic antiinflammatory agents. J Med Chem 27:610–616. https://doi.org/10.1021/jm00371a010
Demopoulos VJ, Rekka E (1995) Isomeric benzoylpyrroleacetic acids: some structural aspects for aldose reductase inhibitory and anti-inflammatory activities. J Pharm Sci 84:79–82. https://doi.org/10.1002/jps.2600840119
Denny WA, Rewcastle GW, Baguley BC (1990) Potential antitumor agents. 59. Structure-activity relationships for 2-phenylbenzimidazole-4-carboxamides, a new class of minimal DNA-intercalating agents which may not act via topoisomerase II. J Med Chem 33:814–819. https://doi.org/10.1021/jm00164a054
Bürli RW, Jones P, McMinn D, Le Q, Duan J-X, Kaizerman JA, Difuntorum S, Moser HE (2004) DNA binding ligands targeting drug-resistant Gram-positive bacteria. Part 2: C-terminal benzimidazoles and derivatives. Bioorg Med Chem Lett 14:1259–1263. https://doi.org/10.1016/j.bmcl.2003.12.043
Srinivasulu V, Sieburth SM, El-Awady R, Kariem NM, Tarazi H, O’Connor MJ, Al-Tel TH (2018) Post-Ugi cascade transformations for accessing diverse chromenopyrrole collections. Org Lett 20:836–839. https://doi.org/10.1021/acs.orglett.7b03986
Arumugam N, Raghunathan R, Almansour AI, Karama U (2012) An efficient synthesis of highly functionalized novel chromeno[4,3-b]pyrroles and indolizino[6,7-b]indoles as potent antimicrobial and antioxidant agents. Bioorg Med Chem Lett 22:1375–1379. https://doi.org/10.1016/j.bmcl.2011.12.061
Purushothaman S, Prasanna R, Niranjana P, Raghunathan R, Nagaraj S, Rengasamy R (2010) Stereoselective synthesis of hexahydro-3-methyl-1-arylchromeno[3,4-b]pyrrole and its annulated heterocycles as potent antimicrobial agents for human pathogens. Bioorg Med Chem Lett 20:7288–7291. https://doi.org/10.1016/j.bmcl.2010.10.073
Buarque CD, Militão GCG, Lima DJB, Costa-Lotufo LV, Pessoa C, de Moraes MO, Cunha-Junior EF, Torres-Santos EC, Netto CD, Costa PRR (2011) Pterocarpanquinones, aza-pterocarpanquinone and derivatives: synthesis, antineoplasic activity on human malignant cell lines and antileishmanial activity on Leishmania amazonensis. Bioorg Med Chem 19:6885–6891. https://doi.org/10.1016/j.bmc.2011.09.025
Pathania S, Rawal RK (2018) Pyrrolopyrimidines: an update on recent advancements in their medicinal attributes. Eur J Med Chem 157:503–526. https://doi.org/10.1016/j.ejmech.2018.08.023
Hilton ST, Ho TCT, Pljevaljcic G, Jones K (2000) A new route to spirooxindoles. Org Lett 2:2639–2641. https://doi.org/10.1021/ol0061642
Rad-Moghadam K, Youseftabar-Miri L (2012) Tetramethylguanidinium triflate: an efficient catalyst solvent for the convergent synthesis of fused spiro[1,4-dihydropyridine-oxindole] compounds. J Fluorine Chem 135:213–219. https://doi.org/10.1016/j.jfluchem.2011.11.007
Li Y, Chen H, Shi C, Shi D, Ji S (2010) Efficient one-pot synthesis of spirooxindole derivatives catalyzed by l-proline in aqueous medium. J Comb Chem 12:231–237. https://doi.org/10.1021/cc9001185
Rad-Moghadam K, Youseftabar-Miri L (2010) Synthesis of novel spiro[dihydropyridine-oxindole] compounds in water. Synlett 2010:1969–1973. https://doi.org/10.1055/s-0030-1258506
Zhang M, Fu Q-Y, Gao G, He H-Y, Zhang Y, Wu Y-S, Zhang Z-H (2017) Catalyst-free, visible-light promoted one-pot synthesis of spirooxindole-pyran derivatives in aqueous ethyl lactate. ACS Sustain Chem Eng 5:6175–6182. https://doi.org/10.1021/acssuschemeng.7b01102
Jossang A, Jossang P, Hadi HA, Sevenet T, Bodo B (1991) Horsfiline, an oxindole alkaloid from Horsfieldia superba. J Org Chem 56:6527–6530. https://doi.org/10.1021/jo00023a016
Elderfield RC, Gilman RE (1972) Alkaloids of Alstonia Muelleriana. Phytochemistry 11:339–343. https://doi.org/10.1016/S0031-9422(00)90012-8
Zhang M, Liu Y-H, Shang Z-R, Hu H-C, Zhang Z-H (2017) Supported molybdenum on graphene oxide/Fe3O4: an efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal Commun 88:39–44. https://doi.org/10.1016/j.catcom.2016.09.028
Jin Q, Zhang J, Jiang C, Zhang D, Gao M, Hu S (2018) Self [3 + 4] cycloadditions of isatin N, N′-cyclic azomethine imine 1,3-dipole with N-(o-chloromethyl)aryl amides. J Org Chem 83:8410–8416. https://doi.org/10.1021/acs.joc.8b01055
Bhaskar G, Arun Y, Balachandran C, Saikumar C, Perumal PT (2012) Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur J Med Chem 51:79–91. https://doi.org/10.1016/j.ejmech.2012.02.024
Ye N, Chen H, Wold EA, Shi P-Y, Zhou J (2016) Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect Dis 2:382–392. https://doi.org/10.1021/acsinfecdis.6b00041
Kumar A, Gupta G, Bishnoi AK, Saxena R, Saini KS, Konwar R, Kumar S, Dwivedi A (2015) Design and synthesis of new bioisosteres of spirooxindoles (MI-63/219) as anti-breast cancer agents. Bioorg Med Chem 23:839–848. https://doi.org/10.1016/j.bmc.2014.12.037
Mishra R, Jana A, Panday AK, Choudhury LH (2019) Synthesis of spirooxindoles fused with pyrazolo-tetrahydropyridinone and coumarin-dihydropyridine-pyrazole tetracycles by reaction medium dependent isatin-based multicomponent reactions. New J Chem 43:2920–2932. https://doi.org/10.1039/C8NJ05465G
Arya AK, Gupta SK, Kumar M (2012) A domino protocol for the efficient synthesis of structurally diverse benzothiazolylquinoline-2,5-diones and their spiro analogues. Tetrahedron Lett 53:6035–6038. https://doi.org/10.1016/j.tetlet.2012.08.099
Arya AK, Kumar M (2011) An efficient green chemical approach for the synthesis of structurally diverse spiroheterocycles with fused heterosystems. Green Chem 13:1332–1338. https://doi.org/10.1039/C1GC00008J
Khandelwal S, Tailor YK, Kumar M (2016) Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J Mol Liq 215:345–386. https://doi.org/10.1016/j.molliq.2015.12.015
Kumar M, Sharma K, Arya AK (2012) Use of SO3H-functionalized halogenfree ionic liquid ([MIM(CH2)4SO3H] [HSO4]) as efficient promoter for the synthesis of structurally diverse spiroheterocycles. Tetrahedron Lett 53:4604–4608. https://doi.org/10.1016/j.tetlet.2012.06.085
Kumar M, Sharma K, Sharma DK, Arya AK (2013) An efficient, ionic liquid mediated one-pot, three component sequential synthesis of 3-benzothiazolyl-2-styrylquinazolin-4(3H)-ones. Tetrahedron Lett 54:878–882. https://doi.org/10.1016/j.tetlet.2012.11.104
Rajawat A, Khandelwal S, Kumar M (2014) Deep eutectic solvent promoted efficient and environmentally benign four-component domino protocol for synthesis of spirooxindoles. RSC Adv 4:5105–5112. https://doi.org/10.1039/C3RA44600J
Tailor YK, Khandelwal S, Kumari Y, Awasthi K, Kumar M (2015) An efficient one pot three-component nanocatalyzed synthesis of spiroheterocycles using TiO2 nanoparticles as a heterogeneous catalyst. RSC Adv 5:46415–46422. https://doi.org/10.1039/C5RA04863J
Tailor YK, Khandelwal S, Gopal R, Rushell E, Prajapati A, Kumar M (2017) Use of nanomagnetic sulfated zirconia (Fe3O4@ZrO2/SO42 −) as sustainable heterogeneous acid catalyst for synthesis of spiroheterocycles under solvent-free conditions. ChemistrySelect 2:11055–11061. https://doi.org/10.1002/slct.201702422
Tailor YK, Khandelwal S, Verma K, Gopal R, Kumar M (2017) Diversity-oriented synthesis of spirooxindoles using surface-modified TiO2 nanoparticles as heterogeneous acid catalyst. ChemistrySelect 2:5933–5941. https://doi.org/10.1002/slct.201700648
Verma K, Tailor YK, Khandelwal S, Agarwal M, Rushell E, Kumari Y, Awasthi K, Kumar M (2018) An efficient and environmentally sustainable domino protocol for the synthesis of structurally diverse spiroannulated pyrimidophenazines using erbium doped TiO2 nanoparticles as a recyclable and reusable heterogeneous acid catalyst. RSC Adv 8:30430–30440. https://doi.org/10.1039/C8RA04919J
Rushell E, Tailor YK, Khandewal S, Verma K, Agarwal M, Kumar M (2019) Deep eutectic solvent promoted synthesis of structurally diverse hybrid molecules with privileged heterocyclic substructures. New J Chem 43:12462–12467. https://doi.org/10.1039/C9NJ02694K
Kulhari A, Khandelwal S, Gupta K, Jain HK, Gopal R, Kumar M (2016) An efficient and environmentally benign diversity-oriented multicomponent synthesis of privileged substructures based spirooxindoles using TiO2 nanoparticles as heterogeneous catalyst. Curr Catal 5:147–160. https://doi.org/10.2174/2211544705666160602123149
Arya AK, Kumar M (2011) Base catalyzed multicomponent synthesis of spiroheterocycles with fused heterosystem. Mol Divers 3:781–789. https://doi.org/10.1007/s11030-011-9309-2
Acknowledgements
We gratefully acknowledge UGC New Delhi for the award of Research Fellowships to Kanchan (SRF) and Esha (SRF). UGC Bhopal is also acknowledged for its financial support. Head, Department of Chemistry is acknowledged for providing Lab and instrumentation facilities in the department.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, K., Tailor, Y.K., Khandelwal, S. et al. Efficient and environmentally sustainable domino protocol for the synthesis of diversified spiroheterocycles with privileged heterocyclic substructures using bio-organic catalyst in aqueous medium. Mol Divers 24, 1355–1365 (2020). https://doi.org/10.1007/s11030-019-09999-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09999-4