Abstract
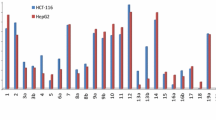
A series of novel \(N\)-(3,4,5-trimethoxyphenyl)pyridin-2(\(1H\))-one derivatives were designed, synthesized, and evaluated for their in vitro cytotoxicity against human colon cancer cells HCT-116. The key steps involved consecutive Chan–Lam- and Buchwald–Hartwig couplings. Most of these C-6 substituted pyridone derivatives showed moderate antiproliferative activity. The preliminary SAR indicated that the conformationally restricted pyridones exhibited more potent cytotoxicity than the flexible counterparts. In addition, cell cycle analysis of the selected compounds 4b and e showed a G2/M arrest, suggesting a possible antitubulin mechanism for these novel pyridone derivatives.





Similar content being viewed by others
References
Tzakos AG, Fokas D, Johannes C, Moussis V, Hatzimichael E, Briasoulis E (2011) Targeting oncogenic protein–protein interactions by diversity oriented synthesis and combinatorial chemistry approaches. Molecules 16:4408–4427. doi:10.3390/molecules16064408
Torres M, Gil S, Parra M (2005) New synthetic methods to 2-pyridone rings. Curr Org Chem 9:1757–1779. doi:10.2174/138527205774610886
Yamazaki T, Yamashita N, Izumi Y, Nakamura Y, Shiota M, Hanatani A, Shimada K, Muro T, Iwao H, Yoshiyama M (2012) The antifibrotic agent pirfenidone inhibits angiotensin II-induced cardiac hypertrophy in mice. Hypertens Res 35:34–40
Nakagawa R, Yoshida H, Asakawa M, Tamiya T, Inoue N, Morita R, Inoue H, Nakao A, Yoshimura A (2011) Pyridone 6, a Pan-JAK inhibitor, ameliorates allergic skin inflammation of NC/Nga mice via suppression of Th2 and enhancement of Th17. J Immunol 187:4611–4620. doi:10.4049/jimmunol.1100649
Li CS, Dixon DD (2004) An efficient copper-catalyzed coupling reaction of pyridin-2-ones with aryl and heterocyclic halides based on Buchwald’s protocol. Tetrahedron Lett 45:4257–4260. doi:10.1016/j.tetlet.2004.04.019
de Candia M, Fossa P, Cellamare S, Mosti L, Carotti A, Altomare C (2005) Insights into structure–activity relationships from lipophilicity profiles of pyridin-2(\(1H\))-one analogs of the cardiotonic agent milrinone. Eur J Pharm Sci 26:78–86. doi: 10.1016/j.ejps.2005.05.001
Kalashnikov VV, Kalashnikova IP, Shestov VI (2008) Synthesis of chalcones on the basis of pyridin-2(\(1H\))-one. Russ J Gen Chem 78:1247–1252. doi: 10.1134/s107036320806025x
Ando M, Sato N, Nagase T, Nagai K, Ishikawa S, Takahashi H, Ohtake N, Ito J, Hirayama M, Mitobe Y, Iwaasa H, Gomori A, Matsushita H, Tadano K, Fujino N, Tanaka S, Ohe T, Ishihara A, Kanatani A, Fukami T (2009) Discovery of pyridone-containing imidazolines as potent and selective inhibitors of neuropeptide Y Y5 receptor. Bioorg Med Chem Lett 17:6106–6122. doi:10.1016/j.bmc.2009.05.069
Morales-Ramos ÁI, Li YH, Hilfiker M, Mecom JS, Eidam P, Shi D, Tseng P-S, Brooks C, Zhang D, Wang N, Jaworski J-P, Morrow D, Fries H, Edwards R, Jin J (2011) Structure–activity relationship studies of novel 3-oxazolidinedione-6-naphthyl-2-pyridinones as potent and orally bioavailable EP3 receptor antagonists. Bioorg Med Chem Lett 21:2806–2811. doi:10.1016/j.bmcl.2011.03.107
Kranjc A, Mašič LP, Reven S, Mikic K, Preželj A, Stegnar M, Kikelj D (2005) Novel pyrazinone and pyridinone thrombin inhibitors incorporating weakly basic heterobicyclic P1-arginine mimetics. Eur J Med Chem 40:782–791. doi:10.1016/j.ejmech.2005.03.007
Ravinder M, Mahendar B, Mattapally S, Hamsini KV, Reddy TN, Rohit C, Srinivas K, Banerjee SK, Rao VJ (2012) Synthesis and evaluation of novel 2-pyridone derivatives as inhibitors of phosphodiesterase3 (PDE3): a target for heart failure and platelet aggregation. Bioorg Med Chem Lett 22:6010–6015. doi:10.1016/j.bmcl.2012.05.019
Li Q, Mitscher LA, Shen LL (2000) The 2-pyridone antibacterial agents: bacterial topoisomerase inhibitors. Med Res Rev 20:231–293. doi:10.1002/1098-1128(200007)20:4<231:aid-med1>3.0.co;2-n
Lv Z, Sheng C, Wang T, Zhang Y, Liu J, Feng J, Sun H, Zhong H, Niu C, Li K (2009) Design, synthesis, and antihepatitis B virus activities of novel 2-pyridone derivatives. J Med Chem 53:660–668. doi:10.1021/jm901237x
Velaparthi U, Liu P, Balasubramanian B, Carboni J, Attar R, Gottardis M, Li A, Greer A, Zoeckler M, Wittman MD, Vyas D (2007) Imidazole moiety replacements in the 3-(1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one inhibitors of insulin-like growth factor receptor-1 (IGF-1R) to improve cytochrome P450 profile. Bioorg Med Chem Lett 17:3072–3076. doi:10.1016/j.bmcl.2007.03.048
Velaparthi U, Wittman M, Liu P, Carboni JM, Lee FY, Attar R, Balimane P, Clarke W, Sinz MW, Hurlburt W, Patel K, Discenza L, Kim S, Gottardis M, Greer A, Li A, Saulnier M, Yang Z, Zimmermann K, Trainor G, Vyas D (2008) Discovery and Evaluation of 4-(2-(4-chloro-1H-pyrazol-1-yl)ethylamino)-3-(6-(1-(3-fluoropropyl)piperidin-4-yl)-4-methyl-1H-benzo[d]imidazol-2 -yl)pyridin-2(\(1H\))-one (BMS-695735), an orally efficacious inhibitor of insulin-like growth factor-1 receptor kinase with broad spectrum in vivo antitumor activity. J Med Chem 51:5897–5900. doi: 10.1021/jm800832q
Wei L, Malhotra SV (2010) Recent development of cyclic amide (pyridone/lactam) moiety containing heterocycles as protein kinase inhibitors. Curr Med Chem 17:234–253. doi:10.1016/j.sbi.2008.09.009
Grattendick KJ (2011) Effects of two anti-TNF-\(\alpha \) compounds: etanercept and 5-ethyl-1-phenyl-2-(\(1H\))-pyridone on secreted and cell-associated TNF-\(\alpha \) in vitro. Pharmacol Pharm 2:238–247. doi:10.4236/pp.2011.24031
Hsieh HP, Liou JP, Mahindroo N (2005) Pharmaceutical design of antimitotic agents based on combretastatins. Curr Pharm Des 11:1655–1677. doi:10.2174/1381612053764751
Shan Y, Zhang J, Liu Z, Wang M, Dong Y (2011) Developments of combretastatin A-4 derivatives as anticancer agents. Curr Med Chem 18:523–538. doi:10.2174/092986711794480221
Marelli M, Conforti F, Statti GA, Cachet X, Michel S, Tillequin F, Menichini F (2011) Biological potential and structure–activity relationships of most recently developed vascular disrupting agents: an overview of new derivatives of natural combretastatin A-4. Curr Med Chem 18:3035–3081. doi:10.2174/092986711796391642
Li Q, Sham HL (2002) Discovery and development of antimitotic agents that inhibit tubulin polymerisation for the treatment of cancer. Expert Opin Ther Pat 12:1663–1702. doi:10.1517/13543776.12.11.1663
Tozer GM, Kanthou C, Parkins CS, Hill SA (2002) The biology of the combretastatins as tumour vascular targeting agents. Int J Exp Pathol 83:21–38. doi:10.1046/j.1365-2613.2002.00211.x
Tseng C-H, Li C-Y, Chiu C-C, Hu H-T, Han C-H, Chen Y-L, Tzeng C-C (2012) Combretastatin A-4 derivatives: synthesis and evaluation of 2,4,5-triaryl-1H-imidazoles as potential agents against H1299 (non-small cell lung cancer cell). Molecular Diversity 16:697–709. doi:10.1007/s11030-012-9396-8
Romagnoli R, Baraldi PG, Pavani MG, Tabrizi MA, Preti D, Fruttarolo F, Piccagli L, Jung MK, Hamel E, Borgatti M, Gambari R (2006) Synthesis and biological evaluation of 2-amino-3-(3\(^{\prime }\),4\(^{\prime }\),5\(^{\prime }\)-trimethoxybenzoyl)-5-aryl thiophenes as a new class of potent antitubulin agents. J Med Chem 49:3906–3915. doi: 10.1021/jm060355e
Beale TM, Myers RM, Shearman JW, Charnock-Jones DS, Brenton JD, Gergely FV, Ley SV (2010) Antivascular and anticancer activity of dihalogenated A-ring analogues of combretastatin A-4. MedChemComm 1:202–208
Mur Blanch N, Chabot GG, Quentin L, Scherman D, Bourg S, Dauzonne D (2012) In vitro and in vivo biological evaluation of new 4,5-disubstituted 1,2,3-triazoles as cis-constrained analogs of combretastatin A4. Eur J Med Chem 54:22–32. doi:10.1016/j.ejmech.2012.04.017
Fardel O, Lecureur V, Guillouzo A (1996) The P-glycoprotein multidrug transporter. Gen Pharmacol Vasc Syst 27:1283–1291. doi:10.1016/s0306-3623(96)00081-x
Wolfe JP, Wagaw S, Buchwald SL (1996) An improved catalyst system for aromatic carbon–nitrogen bond formation: the possible involvement of bis(phosphine) palladium complexes as key intermediates. J Am Chem Soc 118:7215–7216. doi:10.1021/ja9608306
Heo J-N, Song YS, Kim BT (2005) Microwave-promoted synthesis of amino-substituted 2-pyridone derivatives via palladium-catalyzed amination reaction. Tetrahedron Lett 46:4621–4625. doi:10.1016/j.tetlet.2005.04.141
Acknowledgments
This work was supported by the Grants of The National Natural Science Foundation of China (Nos. 81172936 and 21102046) and Grants of The Fundamental Research Funds for the Central Universities. We also thank The Laboratory of Organic Functional Molecules, the Sino-French Institute of ECNU for support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, T., Luo, Y., Sheng, L. et al. Synthesis and in vitro cytotoxic evaluation of novel \(N\)-(3,4,5-trimethoxyphenyl)pyridin-2(\(1H\))-one derivatives. Mol Divers 17, 435–444 (2013). https://doi.org/10.1007/s11030-013-9442-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-013-9442-1