Abstract
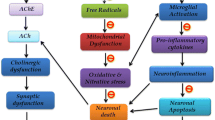
A multifaceted approach can be effective for the treatment of dementia including the most common form, Alzheimer’s disease (AD). However, currently, it involves only symptomatic treatment with cholinergic drugs. Beneficial effects of high Vitamin D3 levels or its intake in the prevention and treatment of cognitive disorders have been reported. Thus, the present study examined the preventive effect of Vitamin D3 (Calcitriol) supplementation on cognitive impairment and evaluated its impact on the accumulation or degradation of Aβ plaques. A single intraperitoneal injection of scopolamine was used to induce cognitive impairment in rats. Treatment of Vitamin D3 was provided for 21 days after the injection. Various behavioral parameters like learning, spatial memory and exploratory behavior, biochemical alterations in the brain homogenate and histology of the hippocampus were investigated. Our results indicated that scopolamine-induced rats depicted cognitive deficits with high Aβ levels and hyperphosphorylated tau proteins in the brain tissue, while Vitamin D supplementation could significantly improve the cognitive status and lower these protein levels. These results were supported by the histopathological and immunohistochemical staining of the hippocampal brain region. Furthermore, mechanistic analysis depicted that Vitamin D supplementation improved the Aβ protein clearance by increasing the neprilysin levels. It also reduced the accumulation of Aβ plaques by lowering neuroinflammation as well as oxidative stress. The present findings indicate that Vitamin D3 supplementation can ameliorate cognitive deficits and thereby delay AD progression by increasing Aβ plaque degradation, reducing inflammation and oxidative stress.








Similar content being viewed by others
Data availability
The data is available with the authors and can be produced on request.
Code availability
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid plaques
- p-Tau:
-
Hyperphosphorylated Tau proteins
- APP:
-
Amyloid precursor protein
- BACE1:
-
β-secretase 1
- NEP:
-
Neprilysin
- IDE:
-
Insulin-degrading enzyme
- MWM:
-
Morris water maze
- ELT:
-
Escape latency time
- AChE:
-
Acetylcholine esterase
- RFU:
-
Relative fluorescence units
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor- α
- IFN-γ:
-
Interferon-γ
- MDA:
-
Malondialdehyde
- GSH:
-
Reduced glutathione
- SOD:
-
Superoxide dismutase
- SEM:
-
Standard error of mean
References
Ahmad W, Ijaz B, Shabbiri K, Ahmed F, Rehman S (2017) Oxidative toxicity in diabetes and Alzheimer's disease: mechanisms behind ROS/ RNS generation. J Biomed Sci 24(1):76. https://doi.org/10.1186/s12929-017-0379-z
Annweiler C, Karras S, Anagnostis P, Beauchet O (2014) Vitamin D supplements: a novel therapeutic approach for Alzheimer patients. Front Pharmacol 5:6. https://doi.org/10.3389/fphar.2014.00006
Banerjee A, Khemka VK, Ganguly A, Roy D, Ganguly U, Chakrabarti S (2015) Vitamin D and Alzheimer's disease: Neurocognition to therapeutics. Int J Alzheimers Dis 2015:192747. https://doi.org/10.1155/2015/192747
Barai P, Raval N, Acharya S, Borisa A, Bhatt H, Acharya N (2019) Neuroprotective effects of bergenin in Alzheimer’s disease: investigation through molecular docking, in vitro and in vivo studies. Behav Brain Res 356:18–40. https://doi.org/10.1016/j.bbr.2018.08.010
Belkhelfa M, Rafa H, Medjeber O, Arroul-Lammali A, Behairi N, Abada-Bendib M, Touil-Boukoffa C (2014) IFN-γ and TNF-α are involved during Alzheimer disease progression and correlate with nitric oxide production: a study in Algerian patients. J Interf Cytokine Res 34(11):839–847. https://doi.org/10.1089/jir.2013.0085
Beydoun MA, Hossain S, Fanelli-Kuczmarski MT, Beydoun HA, Canas JA, Evans MK, Zonderman AB (2018) Vitamin D status and intakes and their association with cognitive trajectory in a longitudinal study of urban adults. J Clin Endocrinol Metab 103(4):1654–1668. https://doi.org/10.1210/jc.2017-02462
Bhuvanendran S, Kumari Y, Othman I, Shaikh MF (2018) Amelioration of cognitive deficit by embelin in a scopolamine-induced Alzheimer's disease-like condition in a rat model. Front Pharmacol 9:665–665. https://doi.org/10.3389/fphar.2018.00665
Bivona G, Lo Sasso B, Gambino CM, Giglio RV, Scazzone C, Agnello L, Ciaccio M (2021) The role of vitamin D as a biomarker in Alzheimer's disease. Brain Sci 11(3):334. https://doi.org/10.3390/brainsci11030334
Bromley-Brits K, Deng Y, Song W (2011) Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp 53:2920. https://doi.org/10.3791/2920
Bruszt N, Bali ZK, Tadepalli SA, Nagy LV, Hernádi I (2021) Potentiation of cognitive enhancer effects of Alzheimer’s disease medication memantine by alpha7 nicotinic acetylcholine receptor agonist PHA-543613 in the Morris water maze task. Psychopharmacology 238:3273–3281. https://doi.org/10.1007/s00213-021-05942-4
Camargo LC, Schöneck M, Sangarapillai N, Honold D, Shah NJ, Langen K-J, Willbold D, Kutzsche J, Schemmert S, Willuweit A (2021) PEAβ triggers cognitive decline and amyloid burden in a novel mouse model of Alzheimer’s disease. Int J Mol Sci 22:7062. https://doi.org/10.3390/ijms22137062
Chai B, Gao F, Wu R, Dong T, Gu C, Lin Q, Zhang Y (2019) Vitamin D deficiency as a risk factor for dementia and Alzheimer's disease: an updated meta-analysis. BMC Neurol 19(1):284. https://doi.org/10.1186/s12883-019-1500-6
Chen ZR, Huang JB, Yang SL, Hong FF (2022) Role of cholinergic signaling in Alzheimer’s disease. Molecules 27:1816. https://doi.org/10.3390/molecules27061816
Conrad CD, Lupien SJ, Thanasoulis LC, McEwen BS (1997) The effects of type I and type II corticosteroid receptor agonists on exploratory behavior and spatial memory in the Y-maze. Brain Res 759(1):76–83. https://doi.org/10.1016/s0006-8993(97)00236-9
Curzon P, Rustay NR, Browman KE (2009) Frontiers in neuroscience: Cued and contextual fear conditioning for rodents. In: Buccafusco JJ (ed) Methods of behavior analysis in neuroscience, 2nd edn. CRC Press/Taylor and Francis Group, LLC, Boca Raton chapter 2
Deb D, Bairy KL, Nayak V, Rao M (2015) Comparative effect of lisinopril and fosinopril in mitigating learning and memory deficit in scopolamine-induced amnesic rats. Adv Pharmacol Sci 2015:521718. https://doi.org/10.1155/2015/521718
Durk MR, Han K, Chow EC, Ahrens R, Henderson JT, Fraser PE, Pang KS (2014) 1α,25-dihydroxyVitamin D3 reduces cerebral amyloid-β accumulation and improves cognition in mouse models of Alzheimer's disease. J Neurosci 34(21):7091–7101. https://doi.org/10.1523/jneurosci.2711-13.2014
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
El-Marasy SA, Abd-Elsalam RM, Ahmed-Farid OA (2018) Ameliorative effect of silymarin on scopolamine-induced dementia in rats. Open Access Maced J Med Sci 6(7):1215–1224. https://doi.org/10.3889/oamjms.2018.257
Farhangi MA, Mesgari-Abbasi M, Nameni G, Hajiluian G, Shahabi P (2017) The effects of vitamin D administration on brain inflammatory markers in high fat diet induced obese rats. BMC Neurosci 18(1):81. https://doi.org/10.1186/s12868-017-0400-1
Feart C, Helmer C, Merle B, Herrmann FR, Annweiler C, Dartigues JF, Delcourt C, Samieri C (2017) Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer's disease in older adults. Alzheimers Dement 13(11):1207–1216. https://doi.org/10.1016/j.jalz.2017.03.003
Gella A, Durany N (2009) Oxidative stress in Alzheimer disease. Cell Adhes Migr 3:88–93
Gorgani S, Jahanshahi M, Elyasi L (2019) Taurine prevents passive avoidance memory impairment, accumulation of amyloid-β plaques, and neuronal loss in the Hippocampus of scopolamine-treated rats. Neurophysiology 51(3):171–179. https://doi.org/10.1007/s11062-019-09810-y
Grimm MOW, Lehmann J, Mett J, Zimmer VC, Grösgen S, Stahlmann CP et al (2014) Impact of vitamin D on amyloid precursor protein processing and amyloid-β peptide degradation in Alzheimer's disease. Neurodegener Dis 13(2–3):75–81. https://doi.org/10.1159/000355462
Grimm MOW, Thiel A, Lauer AA, Winkler J, Lehmann J, Regner L et al (2017) Vitamin D and its analogues decrease amyloid-β (Aβ) formation and increase Aβ-degradation. Int J Mol Sci 18(12):2764. https://doi.org/10.3390/ijms18122764
Hafez HS, Ghareeb DA, Saleh SR, Abady MM, El Demellawy MA, Hussien H, Abdel-Monem N (2017) Neuroprotective effect of ipriflavone against scopolamine-induced memory impairment in rats. Psychopharmacology 234(20):3037–3053. https://doi.org/10.1007/s00213-017-4690-x
Hajjar T, Goh YM, Rajion MA, Vidyadaran S, Li TA, Ebrahimi M (2013) Alterations in neuronal morphology and synaptophysin expression in the rat brain as a result of changes in dietary n-6: n-3 fatty acid ratios. Lipids Health Dis 12:113. https://doi.org/10.1186/1476-511x-12-113
Hamann S, Monarch ES, Goldstein FC (2002) Impaired fear conditioning in Alzheimer's disease. Neuropsychologia 40(8):1187–1195. https://doi.org/10.1016/s0028-3932(01)00223-8
Hanna A, Iremonger K, Das P, Dickson D, Golde T, Janus C (2012) Age-related increase in amyloid plaque burden is associated with impairment in conditioned fear memory in CRND8 mouse model of amyloidosis. Alzheimers Res Ther 4(3):21. https://doi.org/10.1186/alzrt124
Jahn H (2013) Memory loss in Alzheimer's disease. Dialogues Clin Neurosci 15(4):445–454. https://doi.org/10.31887/DCNS.2013.15.4/hjahn
Jayedi A, Rashidy-Pour A, Shab-Bidar S (2019) Vitamin D status and risk of dementia and Alzheimer's disease: a meta-analysis of dose-response. Nutr Neurosci 22(11):750–759. https://doi.org/10.1080/1028415x.2018.1436639
Jha NK, Jha SK, Kar R, Nand P, Swati K, Goswami VK (2019) Nuclear factor-kappa β as a therapeutic target for Alzheimer's disease. J Neurochem 150(2):113–137. https://doi.org/10.1111/jnc.14687
Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F (2019) Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: a randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry 90(12):1347–1352. https://doi.org/10.1136/jnnp-2018-320199
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11(3):151–169. https://doi.org/10.1159/000136485
Jorde R, Mathiesen EB, Rogne S, Wilsgaard T, Kjærgaard M, Grimnes G, Schirmer H (2015) Vitamin D and cognitive function: the Tromsø study. J Neurol Sci 355(1–2):155–161. https://doi.org/10.1016/j.jns.2015.06.009
Joshi P, Turola E, Ruiz A, Bergami A, Libera DD, Benussi L, Giussani P, Magnani G, Comi G, Legname G, Ghidoni R, Furlan R, Matteoli M, Verderio C (2014) Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ 21:582–593. https://doi.org/10.1038/cdd.2013.180
Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-maze for assessment of spatial working and reference memory in mice. In: Guest PC (ed) Pre-clinical models: techniques and protocols, methods in molecular biology. Springer, New York, pp 105–111. https://doi.org/10.1007/978-1-4939-8994-2_10
Kwon SE, Chapman ER (2011) Synaptophysin regulates the kinetics of synaptic vesicle endocytosis in central neurons. Neuron 70:847–854. https://doi.org/10.1016/j.neuron.2011.04.001
Landel V, Annweiler C, Millet P, Morello M, Féron F (2016) Vitamin D, cognition and alzheimer's disease: the therapeutic benefit is in the D-tails. J Alzheimers Dis 53(2):419–444. https://doi.org/10.3233/JAD-150943
Latimer CS, Brewer LD, Searcy JL, Chen KC, Popović J, Kraner SD et al (2014) Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci U S A 111(41):E4359–E4366. https://doi.org/10.1073/pnas.1404477111
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Mahdi O, Baharuldin MTH, Nor NHM, Chiroma SM, Jagadeesan S, Moklas MAM (2019) Chemicals used for the induction of Alzheimer’s disease-like cognitive dysfunctions in rodents. Biomed Res Ther 6(11):3460–3484. https://doi.org/10.15419/bmrat.v6i11.575
Moon JH, Lim S, Han JW, Kim KM, Choi SH, Kim KW, Jang HC (2015) Serum 25-hydroxyVitamin D level and the risk of mild cognitive impairment and dementia: the Korean longitudinal study on health and aging (KLoSHA). Clin Endocrinol 83(1):36–42. https://doi.org/10.1111/cen.12733
Morris RGM (1981) Spatial localization does not require the presence of local cues. Learn Motiv 12(2):239–260. https://doi.org/10.1016/0023-9690(81)90020-5
Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, Ho RC (2018) IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and Meta-analysis. Sci Rep 8:12050. https://doi.org/10.1038/s41598-018-30487-6
Ngatanko Abaissou H, Foyet H, Wado E, Nkwingwa B, Kamda J, Djomessie L et al (2020) Cholinergic-enhancing and antioxidant effect of Vigna subterranea (L.) Verdc. (Fabaceae) landrace aqueous extract on scopolamine-induced amnesia in male swiss mice. Pharm Res 12(3):303–312. https://doi.org/10.4103/pr.pr_62_19
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Rahimzadegan M, Soodi M (2018) Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus. Basic Clin Neurosci 9(1):5–14. https://doi.org/10.29252/NIRP.BCN.9.1.5
Rajashri K, Mudhol S, Serva Peddha M, Borse BB (2020) Neuroprotective effect of spice oleoresins on memory and cognitive impairment associated with scopolamine-induced Alzheimer’s disease in rats. ACS Omega 5(48):30898–30905. https://doi.org/10.1021/acsomega.0c03689
Rodrigues MV, Gutierres JM, Carvalho F, Lopes TF, Antunes V, da Costa P et al (2019) Protection of cholinergic and antioxidant system contributes to the effect of vitamin D3 ameliorating memory dysfunction in sporadic dementia of Alzheimer’s type. Redox Rep 24(1):34–40. https://doi.org/10.1080/13510002.2019.1617514
Sultan S, Taimuri U, Basnan SA, Ai-Orabi WK, Awadallah A, Almowald F, Hazazi A (2020) Low vitamin D and its association with cognitive impairment and dementia. J Aging Res 2020:6097820. https://doi.org/10.1155/2020/6097820
Sun M, Zigman S (1978) An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem 90(1):81–89. https://doi.org/10.1016/0003-2697(78)90010-6
Sun X, Li L, Dong Q-X, Zhu J, Huang Y, Hou S, Yu X, Liu R (2021) Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J Neuroinflammation 18:131. https://doi.org/10.1186/s12974-021-02182-3
Varinthra P, Ganesan K, Huang SP, Chompoopong S, Eurtivong C, Suresh P et al (2021) The 4-(Phenylsulfanyl) butan-2-one improves impaired fear memory retrieval and reduces excessive inflammatory response in triple transgenic Alzheimer's disease mice. Front Aging Neurosci 13:615079. https://doi.org/10.3389/fnagi.2021.615079
Verma A, Goyal A, Kaur R, Kamboj A, Jain UK (2016) Beneficial effect of vitamin D on high-fat diet-induced obesity in wistar rats. Asian J Phar Clin Res 9(4):337–340
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858. https://doi.org/10.1038/nprot.2006.116
Wang WY, Tan MS, Yu JT, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer's disease. Ann Transl Med 3(10):136. https://doi.org/10.3978/j.issn.2305-5839.2015.03.49
World Health Organization WHO (2020) Dementia page. https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed 27 Feb 2021
Yu J, Gattoni-Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni-Celli S, Kindy MS (2011) Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J Alzheimers Dis 25(2):295–307. https://doi.org/10.3233/jad-2011-101986
Zaki HF, Abd-El-Fattah MA, Attia AS (2014) Naringenin protects against scopolamine-induced dementia in rats. Bull Fac Pharm Cairo Univ 52(1):15–25. https://doi.org/10.1016/j.bfopcu.2013.11.001
Zhao Y, Zhao B (2013) Oxidative stress and the pathogenesis of Alzheimer's disease. Oxidative Med Cell Longev 2013:316523. https://doi.org/10.1155/2013/316523
Zheng C, Zhou XW, Wang JZ (2016) The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Transl Neurodegener 5(1):7. https://doi.org/10.1186/s40035-016-0054-4
Zufferey V, Vallet PG, Moeri M, Moulin-Sallanon M, Piotton F, Marin P, Savioz A (2013) Maladaptive exploratory behavior and neuropathology of the PS-1 P117L Alzheimer transgenic mice. Brain Res Bull 94:17–22. https://doi.org/10.1016/j.brainresbull.2013.01.011
Acknowledgements
We are thankful to Institute of Pharmacy, Nirma University for providing infrastructural facilities for carrying out the research work.
Author information
Authors and Affiliations
Contributions
Ms. Parmi Patel contributed to design the experiments, data acquisition and analysis, and manuscript writing. This work is done as a part of her Ph.D. work. Dr. Jigna Shah supervised the Ph.D. work of Ms. Parmi Patel. She contributed to the conceptualization, critical review of the draft, and final approval of the version to be published. All authors read and approved the manuscript.
We confirm herewith our individual participation in the research work and development of this paper in line with the author requirements as per ICMJE. Miss Parmi Patel carried out the literature search, experimental work, data analysis, manuscript preparation and editing. Dr. Jigna Shah defined content, conceptualized and did manuscript review and editing. Further, we declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The Institutional Ethics Committee of Nirma University approved the experimental protocol (protocol approval number IP/PCOL/PHD/23/2018/024 dated 3/8/2018). The study was conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of fisheries, Animal husbandry, and dairying, Govt. of India, New Delhi and National Institute of Health Guide for the Care and Use of Laboratory Animals.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest/competing interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, P., Shah, J. Vitamin D3 supplementation ameliorates cognitive impairment and alters neurodegenerative and inflammatory markers in scopolamine induced rat model. Metab Brain Dis 37, 2653–2667 (2022). https://doi.org/10.1007/s11011-022-01086-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01086-2