Abstract
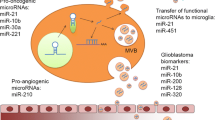
While brain tumors are not extremely frequent, they cause high mortality due to lack of appropriate treatment and late detection. Glioblastoma is the most frequent type of primary brain tumor. This malignant tumor has a highly aggressive behavior. Expression profile of different types of transcripts, methylation status of a number of genomic loci and chromosomal aberrations have been found to affect course of glioblastoma and propensity for recurrence and metastasis. Recent studies have shown that glioblastoma cells produce extracellular vesicles whose cargo can affect behavior of neighboring cells. Several miRNAs such as miR-301a, miR-221, miR-21, miR-16, miR-19b, miR-20, miR-26a, miR-92, miR-93, miR-29a, miR-222, miR-221 and miR-30a have been shown to be transferred by glioblastoma-derived extracellular vesicles and enhance the malignant behavior of these cells. Other components of glioblastoma-derived extracellular vesicles are EGFRvIII mRNA/protein, Ndfip1, PTEN, MYC ssDNA and IDH1 mRNA. In the current review, we discuss the available data about the molecular composition of glioblastoma-derived extracellular vesicles and their impact on the progression of this malignant tumor and its resistance to therapeutic modalities.

Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data Availability
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
Aili Y, Maimaitiming N, Mahemuti Y, Qin H, Wang Y, Wang Z (2021) The Role of Exosomal miRNAs in Glioma: Biological Function and Clinical Application.Frontiers in Oncology,3385
Al-Nedawi K (2008) Intercellular transfer of the oncogenic EGFRv III via tumor cell derived microvesicles. Nat Cell Biol 10:619–624
Al-Nedawi K, Lhotak Meehanbmicallefj, May V, Rak J (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10:619–624
Allahdini F, Reza-Zarei Amirjamshidia, Abdollahi M (2010) Evaluating the prognostic factors effective on the outcome of patients with glioblastoma multiformis: does maximal resection of the tumor lengthen the median survival?World Neurosurg, 73, 128 – 34; discussion e16.
Arscott Wt, Zhao Tandleat, Gordon Sshabasonje, Schlaff Ik, Zhang Cd, Tofilon G, Camphausen Ka (2013) Ionizing radiation and glioblastoma exosomes: implications in tumor biology and cell migration. Translational Oncol 6:638–IN6
Balaj L, Dai Lessardr, Cho L, Pomeroy Y-J, Skog J (2011) Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun 2:1–9
BĂLAȘA A, ȘErban G, Chinezu R, HurghiȘ C, TĂMAȘ F, Manu D (2020) The Involvement of Exosomes in Glioblastoma Development, Diagnosis, Prognosis, and Treatment. Brain sciences, 10, 553
Batista Bs, Eng Ws, Hendricks-MuÑOz Pilobellokt, Mahal Lk (2011) Identification of a conserved glycan signature for microvesicles. J Proteome Res 10:4624–4633
Chen Ww, Balaj L, Liau Lm, Samuels Ml, Maguire Kotsopoulossk, Soto Caloguidicel, Garrett H, Zhu Ld (2013) BEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol Therapy-Nucleic Acids 2:e109
Chistiakov Da, Chekhonin Vp (2014) Extracellular vesicles shed by glioma cells: pathogenic role and clinical value. Tumor Biology 35:8425–8438
Chuang H-Y, Su Y-K, Chen Liuh-W, Cho C-Hchius-C, Chen D-Ylins-Z, Lin C-M (2019) Preclinical evidence of STAT3 inhibitor pacritinib overcoming temozolomide resistance via downregulating miR-21-enriched exosomes from M2 glioblastoma-associated macrophages. J Clin Med 8:959
D’Asti E, Huang A, Kool M, Meehan B, Jabado Chanja, Rak J (2016) Tissue factor regulation by miR-520 g in primitive neuronal brain tumor cells: a possible link between oncomirs and the vascular tumor microenvironment. Am J Pathol 186:446–459
D’Souza-Schorey C, Clancy Jw (2012) Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev 26:1287–1299
D’Asti E, Huang A, Rak J (2012) Downregulation of tissue factor (TF) in medulloblastoma cells expressing miR-520 g. Proceedings of Keystone Syposia
Dai J, Su Y, Zhong S, Cong L, Liu B, Tao Yangj, He Y, Chen Z, Jiang Y (2020) Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther 5:145
De Vrij J, Maas Sn, Kwappenberg Km, Dekker Schnoorrkleijna, Luider L, De Tm, Witte Ld, Litjens M (2015) & VAN STRIEN, M. E. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. International journal of cancer, 137, 1630–1642
Esmaeili M, Hosseini Niazivpourfathollahaa, Nakhlestani Mkm, Taheri Mgolzadehk, Ghafouri-Fard M, Atarodi K (2019) The impact of parathyroid hormone treated mesenchymal stem cells on ex-vivo expansion of cord blood hematopoietic stem cells. Gene Rep 17:100490
Felsberg J, Rapp M, Fimmers Loesers, Stummer R, Goeppert W, Friedensdorf Msteigerhj, Reifenberger B, Sabel Mc (2009) Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res 15:6683–6693
Gallego O (2015) Nonsurgical treatment of recurrent glioblastoma. Curr Oncol 22:e273–e281
Ghafouri-Fard S, Abak Agabalazadeha, Shoorei A, Hassanzadeh Taheri H, Taheri Mm, Sharifi G 2021a. Role of Long Non-Coding RNAs in Conferring Resistance in Tumors of the Nervous System.Front Oncol, 11,670917
Ghafouri-Fard S, Abak Glassymc, Hussen A, Niazi Bm, Taheri M (2021b) The interaction between miRNAs/lncRNAs and Notch pathway in human disorders. Biomed Pharmacother 138:111496
Ghafouri-Fard S, Niazi V, Taheri M (2020a) Role of miRNAs and lncRNAs in hematopoietic stem cell differentiation. Non-coding RNA Research
Ghafouri-Fard S, Niazi V, Taheri M (2020b) Role of miRNAs in conveying message of stem cells via extracellular vesicles.Experimental and Molecular Pathology,104569
Giusti I, Delle Monache S, Di Francesco M, SanitÀ P, D’Ascenzo S, Dolo V (2016) From glioblastoma to endothelial cells through extracellular vesicles: messages for angiogenesis. Tumor Biology 37:12743–12753
Graner Mw, Dechkovskaia Alzateo, Bigner Dd (2009) Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 23:1541–1557
Guescini M, Stocchi Genedanis, Agnati Lf (2010) Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm 117:1–4
Hellwinkel Je, Harland Redzicjs, Gunaydin Ta, Anchordoquy D, Graner Mw (2015) Glioma-derived extracellular vesicles selectively suppress immune responses. Neurooncology 18:497–506
Kalluri R, Lebleu Vs (2020) The biology, function, and biomedical applications of exosomes.Science,367
Kucharzewska P, Svensson Christiansonhcwelchje, RingnÉR Kjfredlunde, MÖRgelin M, Bourseau-Guilmain M, Belting M (2013) Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proceedings of the National Academy of Sciences, 110, 7312–7317
Lan F, Pan Qingq, Yu Qhum, Yue X (2018) Serum exosomal miR-301a as a potential diagnostic and prognostic biomarker for human glioma. Cell Oncol (Dordr) 41:25–33
Litak J, Mazurek M, Kamieniak Grochowskic, RoliŃSki J (2019) PD-L1/PD-1 Axis in Glioblastoma Multiforme. Int J Mol Sci 20:5347
Liu Y, Shete S, Hosking F, Robertson L, Houlston R, Bondy M (2010) Genetic advances in glioma: susceptibility genes and networks. Curr Opin Genet Dev 20:239–244
Mallawaaratchy Dm, Hallal S, Ly Russellb, Ebrahimkhani L, Wei S, Kaufman Kl (2017) Comprehensive proteome profiling of glioblastoma-derived extracellular vesicles identifies markers for more aggressive disease. J Neurooncol 131:233–244
Naryzhny S, Kopylov Volnitskiya, Zorina A, Kamyshinsky E, Bairamukov R, Shtam T (2020) Proteome of Glioblastoma-Derived Exosomes as a Source of Biomarkers. Biomedicines, 8, 216
Niazi V, Ahani Parsehb, Karami M, Gilanchi F, Atarodi S, Soufi K, Ghafouri-Fard Msoleimanim, Taheri M (2020) Communication between stromal and hematopoietic stem cell by exosomes in normal and malignant bone marrow niche. Biomed Pharmacother 132:110854
Pinet S, Bessette B, Vedrenne N, Battu Lacroixarichardljauberteaum-O, LallouÉ F (2016) TrkB-containing exosomes promote the transfer of glioblastoma aggressiveness to YKL-40-inactivated glioblastoma cells. Oncotarget 7:50349
Putz U, Goh Howittjdoana, Silke C-Plowl-H, Tan S-S (2012) The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal 5:ra70–ra70
Qian M, Guo Wangs, Zhang Xwangj, Gao Zqiuw, Chen X, Xu Z, Zhao R (2020) Hypoxic glioma-derived exosomes deliver microRNA-1246 to induce M2 macrophage polarization by targeting TERF2IP via the STAT3 and NF-κB pathways. Oncogene 39:428–442
Rezaei O, Taheri Tamizkarkhsharifig, Ghafouri-Fard S (2020) Emerging Role of Long Non-Coding RNAs in the Pathobiology of Glioblastoma. Front Oncol 10:625884
Ricklefs F, Mineo M, Nakano Roojak, Charest I, Breakefield Aweisslederr, Godlewski Xochioccaea, Bronisz A (2016) Extracellular vesicles from high-grade glioma exchange diverse pro-oncogenic signals that maintain intratumoral heterogeneity. Cancer Res 76:2876–2881
Roth P, Weller M (2014) Challenges to targeting epidermal growth factor receptor in glioblastoma: escape mechanisms and combinatorial treatment strategies. Neurooncology 16:viii14–viii19
Sathornsumetee S, Rich Jn (2006) New approaches to primary brain tumor treatment. Anticancer Drugs 17:1003–1016
Setti M, Osti D, Ortensi Richichic, Del B, Bene M, Beznoussenko Fornasaril, Mironov G, Rappa A, Cuomo A (2015) Extracellular vesicle-mediated transfer of CLIC1 protein is a novel mechanism for the regulation of glioblastoma growth. Oncotarget 6:31413
Seystahl K, Tritschler I, Szabo E, Tabatabai G, Weller M (2015) Differential regulation of TGF-β–induced, ALK-5–mediated VEGF release by SMAD2/3 versus SMAD1/5/8 signaling in glioblastoma. Neurooncology 17:254–265
Shao H, Lee Chungj, Balaj K, Min L, Carter C, Hochberg Bs, Lee Fhbreakefieldxo, Weissleder R (2015) Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun 6:1–9
Skog J, Wurdinger T, Van Rijn S, Meijer D, Sena-Esteves Gainchel, Curry W Jr, Carter Bs, Krichevsky Am, Breakefield Xo (2008a) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers.Nat Cell Biol, 10,1470–1476
Skog J, WÜRdinger T, Van Rijn S, Meijer Dh, Curry Gainchel, Krichevsky Wtcarterbs, A. M. & Breakefield, X. O (2008b) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476
Sun X, Zhao Maxwangj, Wang Y, Chen Ybihljc, Jiang C (2017) Glioma stem cells-derived exosomes promote the angiogenic ability of endothelial cells through miR-21/VEGF signal. Oncotarget 8:36137
Sung H, Siegel Ferlayj, Laversanne Rl, Jemal Msoerjomatarami, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 71:209–249
Taylor Og, Skelding Ka (2019) Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front Oncol 9:963
Thuringer D, Chanteloup G, Pernet Boucherj, Jego Nboudescoc, Chatelier G, Bois A, Cronier L (2017) Modulation of the inwardly rectifying potassium channel Kir4. 1 by the pro-invasive miR-5096 in glioblastoma cells. Oncotarget 8:37681
Treps L, Edmond S, Harford-Wright E, Galan-Moya E, Schmitt A, Azzi S, Bidere Citernea, Ricard N, Gavard J (2016) Extracellular vesicle-transported Semaphorin3A promotes vascular permeability in glioblastoma. Oncogene 35:2615–2623
Uribe D, Niechi TorresÁRochajd, Sobrevia IoyarzÚNc, San L, MartÍN R, Quezada C (2017) Multidrug resistance in glioblastoma stem-like cells: Role of the hypoxic microenvironment and adenosine signaling. Mol Aspects Med 55:140–151
Vaidya M, Sugaya K (2020) DNA Associated with Circulating Exosomes as a Biomarker for Glioma. Genes 11:1276
Van Der Vos Ke, Zhang Abelser, Carrizosa Xlaic, Oakley E, Skog J (2015) Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neurooncology 18:58–69
Vienne-Jumeau A, Tafani C, Ricard D (2019) Environmental risk factors of primary brain tumors: A review. Rev Neurol (Paris) 175:664–678
Vogelbaum Ma (2012) Does extent of resection of a glioblastoma matter? Clin Neurosurg 59:79–81
Wang B, Wu Z-H, Chai Loup-Y, Han C, Ning S-Y, Li M (2019) Human bone marrow-derived mesenchymal stem cell-secreted exosomes overexpressing microRNA-34a ameliorate glioblastoma development via down-regulating MYCN. Cell Oncol 42:783–799
Wu X, Wang X, Hao Wangj, Liu Y, Wang F, Yang X, Lu Z (2021) The Roles of Exosomes as Future Therapeutic Agents and Diagnostic Tools for Glioma.Frontiers in Oncology,11
Yang J-K, Yang J-P, Tong J, Jing S-Y, Wang Fanb, Jiao B-H (2017) Exosomal miR-221 targets DNM3 to induce tumor progression and temozolomide resistance in glioma. J Neurooncol 131:255–265
Yue X, Lan F, Xia T (2019) Hypoxic glioma cell-secreted exosomal miR-301a activates Wnt/β-catenin signaling and promotes radiation resistance by targeting TCEAL7. Mol Ther 27:1939–1949
Zeng A, Wei Z, Huang Yanwyinj, Li Xzhoux, Shen R, Wu F, Wang X (2018) Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett 436:10–21
Zhang Y, Liu Y, Liu H, Tang Wh (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19
Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Sampath D (2012) Tumour-secreted miR‐9 promotes endothelial cell migration and angiogenesis by activating the JAK‐STAT pathway. EMBO J 31:3513–3523
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SGF wrote the manuscript and revised it. MT and BMH supervised and designed the study. VN, MS and MK collected the data and designed the figures and tables. All authors read and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participant
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent forms were obtained from all study participants. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences. All methods were performed in accordance with the relevant guidelines and regulations.
Competing interests
The authors declare they have no conflict of interest.
Consent of publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khayamzadeh, M., Niazi, V., Hussen, B.M. et al. Emerging role of extracellular vesicles in the pathogenesis of glioblastoma. Metab Brain Dis 38, 177–184 (2023). https://doi.org/10.1007/s11011-022-01074-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01074-6