Abstract
Schizophrenia is one of the most disabling mental disorders that affects up to 1 % of the population worldwide. Although the causes of this disorder remain unknown, it has been extensively characterized by a broad range of emotional, ideational and cognitive impairments. Studies indicate that schizophrenia affects neurotransmitters such as dopamine, glutamate and acetylcholine. Recent studies suggest that rivastigmine (an acetylcholinesterase inhibitor) is important to improve the cognitive symptoms of schizophrenia. Therefore, the present study evaluated the protective effect of rivastigmine against the ketamine-induced behavioral (hyperlocomotion and cognitive deficit) and biochemical (increase of acetylcholinesterase activity) changes which characterize an animal model of schizophrenia in rats. Our results indicated that rivastigmine was effective to improve the cognitive deficit in different task (immediate memory, long term memory and short term memory) induced by ketamine in rats. Moreover, we observed that rivastigmina reversed the increase of acetylcholinesterase activity induced by ketamine in the cerebral cortex, hippocampus and striatum. However, rivastigmine was not able to prevent the ketamine-induced hyperlocomotion. In conslusion, ours results indicate that cholinergic system might be an important therapeutic target in the physiopathology of schizophrenia, mainly in the cognition, but additional studies should be carried.
Similar content being viewed by others
Introduction
Schizophrenia is one of the most severe and disabling psychiatric disorders that affects up to 1 % of the population worldwide and it causes an enormous social and economic impact (Mueser and Penn 2004). The physiopathology of the disease is poorly understood and is usually associated with the incidence of comorbidities. The affected individuals have a profound disturbance of mental functions, emotions and behavior, which disrupts many of the most basic human processes of perception and judgment. The clinical symptoms include positive symptoms such as hallucinations and delusions, negative symptoms as anhedonia, avolition and social withdrawal and cognitive symptoms like memory and attention impairment (Meyer and Feldon 2010). Currently, pharmacological therapy with antipsychotics is able to control the positive and negative symptoms, however the effects are still limited regarding cognitive function. Furthermore, most drugs generates neurological and physical side effects which hinders the adherence to the treatment and consequently induces relapses of symptoms (Schultz et al. 2007).
Dopamine plays an important role in the schizophrenia and almost all antipsychotics alter dopaminergic neurotransmission (Fredman, 20003). However, dopaminergic system cannot entirely explain the pathophysiology of schizophrenia, and the role of other neurotransmitters, such as acetylcholine (ACh), has been investigating (Schultz et al. 2007).
Acetylcholine (ACh) is a neurotransmitter that acts at both peripheral and central nervous system (CNS) of many organisms. The signaling of ACh modulates synaptic plasticity, particularly when related to learning and short-term memory (Woolf and Butcher 1986; Perry et al. 1999). This neurotransmitter is hydrolysed into synaptic cleft by acetylcholinesterase (AChE), an enzyme which is a key regulator of cholinergic transmission (Azevedo et al. 2011). Also, alterations in ACh levels are related with cognitive dysfunction in schizophrenia (Meltzer and McGurk 1999). In this context, some studies indicate that nicotine (agonist of nicotinic ACh receptor) improved the negative symptoms and cognitive performance in patients with schizophrenia (Barr et al. 2008; Jubelt et al. 2008). This fact possibly justifies the higher rates of smoking in this population comparing to general subjects (Hughes et al. 1986). Therefore, one of the challenges of the studies related to schizophrenia is to perform clinical research in order to improve the characteristics of the crippling disease, as well as the development of experimental research with the objective to find the best therapeutic targets.
Acetylcholinesterase inhibitors (ChEI) are useful for improving cognitive function in Alzheimer disease by increasing ACh levels into synaptic cleft. These drugs are also being tested in schizophrenic patients for cognitive improvement in schizophrenia (Ribeiz et al. 2010). Furthermore, several ChEI are available for clinical trials as tacrine, donepezil, rivastigmine and galantamine and they are also effective in Alzheimer’s disease (Rogers et al. 1998; Rogers and Kashima 1998; Rösler et al. 1999; Tariot et al. 2000). In particular, some studies indicate that rivastigmine may be an important therapeutic strategy to improve the cognitive deficits in schizophrenic patients, but confirmatory studies are needed to determine the clinical utility of this drug (Ribeiz et al. 2010).
Animal models provide an opportunity to discover new targets for the treatment of schizophrenia. Accordingly, the animal model of ketamine is one of the most widely accepted since its generates behavioral (schizophrenia-like positive, negative and cognitive symptoms) and biochemical alterations in animals, similar to human schizophrenia (Becker et al. 2003; Chatterjee and Khanna 2011; Adell et al. 2012; Gilmour et al. 2012). Take into account that N-Methyl-D-aspartate (NMDA) receptor hypofunction is observed in the schizophrenia (Olney et al. 1999), this animal model induces similar alteration, since ketamine is a non-competitive antagonist of NMDA receptor, an ionotropic glutamate receptor (Bressan and Pilowsky 2003; Adell et al. 2012; Gilmour et al. 2012). Many studies showed that ketamine administration induces schizophrenia-like behavioral changes (hyperlocomotion, deficit cognitive, stereotype) in rodents, indicating a useful animal model of schizophrenia (Becker et al. 2003; Becker and Grecksch 2004; de Oliveira et al. 2009; de Oliveira et al. 2011; Canever et al. 2010; Fraga et al. 2011; Chatterjee and Khanna 2011; Adell et al. 2012; Gilmour et al. 2012).
Considering that: (i) Schizophrenia is a psychiatric disorder complex, (Meyer and Feldon 2010), (ii) The discovery of new non-dopaminergic targets effective in reducing the symptoms is needed (Kane and Correll 2010), (iii) Ketamine administration induces schizophrenia-like behavioral Alterations in rats (Becker et al. 2003; Becker and Grecksch 2004; Oliveira et al. 2009; Canever et al. 2010; Chatterjee et al. 2,011), (iv) There is evidence that rivastigmine may be an important therapeutic target for improvement of cognitive function in schizophrenic patients (Ribeiz et al. 2010), we induced an animal model of schizophrenia through chronic administration of ketamine in sub-anesthetic doses and test different behavioral parameters in rats treated with rivastigmine.
Materials and methods
Animals
Adult male Wistar rats (250–300 g) were obtained from the Central Animal House of Universidade do Extremo Sul Catarinense (UNESC). They were caged in groups of five with free access to food and water, and were maintained on a 12-h light–dark cycle (lights on at 7:00 am), at a temperature of 23 ± 1 °C. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care, with the approval of UNESC Ethics Committee.
Drugs
Ketamine (CU Chemie Uetikon, Germany), Rivastigmine (Novartis Pharma, Brazil) were directly dissolved in saline (Sal) solution (NaCl 0.9 %, w/v). The doses of ketamine (25 mg/kg, i.p., once a day for 14 days) and rivastigmine (0.5 mg/kg, i.p., once a day for 7 days) were based on previous studies performed by de Oliveira et al. (2011) and Comim et al. (2009), respectively.
Animal model of schizophrenia
The animals received one injection of ketamine (CU Chemie Uetikon, Germany) 25 mg/kg (i.p., once a day) or saline (vehicle) for 14 days. Between the 8th and the 14th day, the animals received rivastigmine (0.5 mg/kg, i.p.) or saline (vehicle), that is, during this period two types of treatment were performed in animals. This experimental design led four experimental groups, as detailed below: saline/saline; saline/rivastigmine; ketamine/saline; ketamine/rivastigmine. Rivastigmine was administered 2 h after ketamine to prevent interaction between the drugs.
Behavioral tests were done on the 15th, 16th and 17th days. The animals received a single injection of ketamine or saline and locomotor activity (after 30 min from the injection) was assessed during 15 min using a proper apparatus. These rats were killed by decapitation immediately after the locomotor activity and prefrontal cortex, hippocampus and striatum were manually dissected on ice, rapidly frozen on dry ice and stored at −70 °C for posterior analysis of acetylcholinesterase activity. On the 16th day (24 h after the last injection of ketamine or saline), an independent group of animals were used for evaluate the behavioral in the inhibitory avoidance task (16th and 17th). For a better understanding of the experimental design see Fig. 1.
Diagram of the complete experimental schedule. The animals received one injection of ketamine 25 mg/kg (i.p., once a day) or saline (vehicle) for 14 days. Between the 8th and the 14th day, the animals received rivastigmine (0.5 mg/kg, i.p., once a day for 7 days) or saline (vehicle). On the 15th day, the animals received a single injection of ketamine or saline and 30 min after this treatment, the locomotor activity was assessed using the locomotor activity chamber for 15 min. Immediately after locomotor activity, these rats were killed by decapitation and prefrontal cortex, hippocampus and striatum were removed for analyses of acetylcholinesterase activity. An independent group of animals (24 h of last injection of ketamine or saline) was used for evaluate the behavioral in the inhibitory avoidance task (16th and 17th)
Open-field test
We assessed locomotor activity using the open-field test. The test was performed in a 50 × 25 × 50 cm arena and locomotor activity was measured for 15 min using a computerized system (Activity Monitor—Insight laboratory equipment, Ribeirão Preto, SP). This equipment monitored locomotor activity via the covered distance (cm) by the animal, dividing the evaluation time into blocks of 5 min. Each animal was constantly monitored by a system installed in the arena containing six parallel bars, each of which contained 16 infrared sensors that detect the rat’s exact position and movement. This system allows a detailed analysis of each animal’s behavior. Information detected by the sensors is transmitted to a computer in which the animal’s activity is recorded each 5 min by a dedicated program (database: Open Source version Interbase 6.01). The covered distance by the animal is considered to be the sum of the changes in position monitored by the activity arena; the software calculates the distance between two locations, plus the previously traveled distances.
Inhibitory avoidance
The inhibitory avoidance evaluation was initiated 24 h after the last injection, with 60 rats, divided into 4 groups (saline/saline; saline/rivastigmine; ketamine/saline; ketamine/rivastigmine). The apparatus consisted of an acrylic box with parallel stainless steel bars and a platform placed on the floor (Quevedo et al. 1997; Roesler et al. 2003). In the training session rats were placed on the platform and we measured their latency to step down with all four paws. Immediately after step down from the platform, the animals received a 0.4 mA footshock (electrical shock induced through the feet) for 2 s.
In the test session, animals were again placed on the platform and had their latency to step down on the grid measured, except that no footshock was given. Latency is a classic parameter for memory retention tasks. Test sessions for inhibitory avoidance were conducted immediately after training (5 s) to evaluate immediate memory (Barros et al. 2005). The given interval between training and test sessions was 1.5 h to evaluate short-term memory (Izquierdo and Medina 1998; Bevilaqua et al. 2003) and 24 h to assessed long-term memory (Bevilaqua et al. 2003; Lima et al. 2005).
Acetylcholinesterase activity
After the evaluation of locomotor activity, animals were killed by decapitation and brain structures were rapidly dissected. Prefrontal cortex, striatum and hippocampus were homogenate and prepared to the assay.
AChE activity was assayed according to the method of Ellman et al. (1961). The reaction mixture (2 ml final volume) contained 100 mM K + −phosphate buffer (pH 7.5) and 1 mM 5,5′-dithiobisnitrobenzoic acid. The method is based on the formation of the yellow anion, 5,5′-dithio-bis-acid-nitrobenzoic, which is measured by absorbance at 412 nm during a 2-min incubation at 25 °C. The enzyme (40–50 μg of protein) was preincubated for 2 min. The reaction was initiated by adding 0.8 mM acetylthiocholine iodide (AcSCh). All samples were run in duplicate or triplicate, and the enzyme activity is expressed in micromoles AcSCh per hour per milligram of protein.
Protein determination
Protein was measured using the method described by Lowry et al. (1951) using bovine serum albumin as standard.
Statistical analysis
All experimental results are given as the mean ± SEM. Comparisons between experimental and control groups were performed by two-way ANOVA followed by Newman-Keuls test when appropriate. A value of p <0.05 was considered to be significant. Training-test session latency differences were assessed by the Wilcoxon test followed by individual Mann–Whitney U-test. A value of p < 0.05 was considered to be significant.
Results
Figure 2 shows the results related the memory of animals. We tested immediate memory, short and long-term memory consolidation. Statistical analysis indicates that no differences among groups were observed in training session. Differently, saline (control) or rivastigmine treated rats showed improvement of test session latency, when compared to training session, indicating that animals adequately learned the task (Wilcoxon test, p < 0.05). Ketamine impaired memory in all test session performance, as showed by Mann–Whitney U-tests, p < 0.05, (Fig. 2 [immediate memory, Mann–Whitney U = 0.5, p < 0.001; short term memory, Mann–Whitney U = 12, p < 0.001; long term memory, Mann–Whitney U = 37.5, p < 0.001]) when compared with control group.
Effect of the rivastigmine (0.5 mg/kg, i.p./day for 7 days) on differents task of memory (imediate memory, short and long term memory) in a ketamine-induced animal model of schizophrenia (25 mg/kg, i.p./day for 7 days) in rats. Data are expressed as median ± interquartile range, n = 15. * P < 0.05 when compared to training of all experimental groups. # P < 0.05 when compared to control group (Saline/Saline) of each type of memory, respectively. & P < 0.05 when compared to ketamine/saline group of each type of memory, respectively
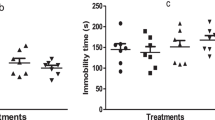
In respect to locomotor activity, the animals treated with rivastigmine alone, for 7 days, did not show alteration of covered distance. Animals that received ketamine injections for fourteen consecutive days presented a significant increase in locomotor activity when compared to animals that received saline. However, animals treated with ketamine + rivastigmine presented an increase in locomotor activity showing that rivastigmine has no effect on this parameter (Fig. 3). The two-way ANOVA revealed a significant effect of ketamine pretreatment [F(1,56) = 38.31, p < 0.01], but not of rivastigmine treatment [F(1,56) = 0.27, p = 0.60] and interaction between rivastigmine vs. ketamine [F(1,56) = 0.31, p = 0.58].
Effect of the rivastigmine (0.5 mg/kg, i.p./day for 7 days) on locomotor activity in a ketamine-induced animal model of schizophrenia (25 mg/kg, i.p./day for 7 days) in rats. Data were analyzed by two-way ANOVA followed by Newman-Keuls test when appropriated. Values are expressed as mean ± S.E.M. (n = 15). ** P < 0.01 when compared to saline/saline group
We also studied the effect of ketamine and rivastigmine on AChE activity in the prefrontal cortex, hippocampus and striatum. As we already expected, rivastigmine alone inhibited AChE activity and ketamine significantly increased the enzyme activity. It was also observed that rivastigmine could reverse the effect caused by ketamine, as compared to control group (Fig. 4). The two-way ANOVA revealed a significant effect in the prefrontal cortex (ketamine pretreatment [F(1,12) = 68.19, p < 0.01], rivastigmine treatment [F(1,12) = 61.86, p < 0.01] and interaction between pretreatment vs. treatment [F(1,12) = 8.35, p < 0.05]), hippocampus (ketamine pretreatment [F(1,16) = 109.15, p < 0.01], rivastigmine treatment [F(1,16) = 82.64, p < 0.01] and interaction between pretreatment vs. treatment [F(1,16) = 11.60, p < 0.01]) and striatum (ketamine pretreatment [F(1,15) = 49.50, p < 0.01], rivastigmine treatment [F(1,15) = 55.21, p < 0.01] and interaction between pretreatment vs. treatment [F(1,15) = 6.13, p < 0.05]).
Effect of the rivastigmine (0.5 mg/kg, i.p./day for 7 days) on acetylcholinesterase (AChE) activity in a ketamine-induced animal model of schizophrenia (25 mg/kg, i.p./day for 7 days) in prefrontal cortex, hippocampus and striatum of rats. Data were analyzed by two-way ANOVA followed by Newman-Keuls test when appropriated. Values are expressed as mean ± S.E.M. (n = 4–6) && P < 0.01 when compared to saline/saline group, it is indicating decrease when compared to saline/saline group. ** P < 0.01 when compared to saline/saline group, it is indicating increase when compared to saline/saline group. # P < 0.01 compared to ketamine/saline group, it is indicating reversion when compared to ketamine/saline group
Discussion
The results of present study showed that the rivastigmine was effective to improve the cognitive deficit in different task (immediate memory, long term memory and short term memory) induced by ketamine in rats. Moreover, we observed that the increase of acetylcholinesterase (AChE) activity induced by ketamine in the prefrontal cortex, hippocampus and striatum, was reversed by rivastigmine. However, rivastigmine was not able to prevent the ketamine-induced hyperlocomotion.
Glutamatergic (NMDA receptors) hypofunction induced by ketamine (non-competitive NMDA receptor antagonist) is an important animal model of schizophrenia, which have been utilized to try to understand this complex disease and to evaluate the therapeutic potential of candidate target for antipsychotics. This animal model induces positive (hyperlocomotion and stereotype), negative (social deficits) and cognitive (cognitive deficit) symptoms (Neill et al. 2010; Adell et al. 2012; Gilmour et al. 2012). Those results were also observed by our group and other researches group (Becker et al. 2003; Becker and Grecksch 2004; Chatterjee and Khanna 2011). In agreement, in this work, ketamine induced hyperlocomotion, cognitive deficit and biochemical alterations (de Oliveira et al. 2009; de Oliveira et al. 2011; Canever et al. 2010; Fraga et al. 2011).
Although dopamine has been considered the key neurotransmitter involved in the pathophysiology of schizophrenia (Barch and Ceaser 2012; Howes et al. 2012), many studies have established that the cholinergic system (muscarinic and nicotinic receptors) is important for neuromodulation of cognitive processes in schizophrenia, since this disorder is associated with a range of cognitive deficits (Davis et al. 1975; Powchik et al. 1998; Sarter et al. 2012). Furthermore, it is well known that classical antipsychotics do not improve the cognitive deficits in both schizophrenic patients and pre-clinical models of schizophrenia (Neill et al. 2010). Therefore, in the present study we used the rivastigmine, an AChE inhibitor widely used in the treatment of Alzheimer disease, for evaluating the possible beneficial effect in enhancing cognitive functions in the animal model of schizophrenia induced by ketamine in rats.
The results from behavior analysis indicate that rivastigmine did not reverse the ketamine-induced hyperlocomotion. These results indicated that the behavioral effect of rivastigmine is specific to cognition (cognitive symptoms of schizophrenia), but not to locomotion (positive symptoms of schizophrenia). It is important emphasize that the cholinergic system is closely involved with the cognition, since that drugs that enhance cholinergic neural transmission are well known for pro-cognitive properties (Singh et al. 2012; Yakel 2013). Thus, it is expected that inhibitors of acetylcholinesterase activity does not induce reversal of hyperlocomotion induced by ketamine. Our data corroborate with a study performed by Csernansky et al. (2005) which also showed that AChE inhibitor (donepezil and galantamine) did not reversed the hyperlocomotion produced by MK-801, an animal model of schizophrenia performed in mice.
Our results also indicate that rivastigmine was effective to improve the cognitive deficit on different task (immediate memory, short term and long term memory) induced by ketamine. Supporting our study, evidences indicated that AChE inhibitor increase ACh availability at synaptic cleft, consequently, the therapeutic effects of these drugs are related to cholinergic stimulation in the brain structures involved in the regulation of cognitive and behavioral processes (Nieoullon 2010). Ours results are in accordance with findings which suggest that rivastigmine improves the cognitive function in schizophrenic patients (Lenzi et al. 2003; Aasen et al. 2005; Kumari et al. 2006). An interesting meta-analysis of the literature performed Ribeiz et al. (2010) indicated that specific cognitive deficits (memory, and the motor speed and attention part of executive function) of patients with schizophrenia and schizoaffective disorder are responsive to rivastigmine, donepezil and galantamine as adjunctive therapy.
Moreover, the present study showed that chronic ketamine administration increase degradation of ACh in the prefrontal cortex, hippocampus and striatum. These brain structures are closely related to behavior and biochemical alterations in schizophrenia (Tzschentke and Schmidt 2000; Cardinal et al. 2002; Sesack and Carr 2002; Lodge and Grace 2011; Wolf et al. 2011). In this line, a study performed by Karson et al. (1998) showed that cognitive impairment was correlated with reduction of mesopontine choline acetyltransferase (ChAT) activity, analyzed in postmortem brain of schizophrenic patients. This enzyme is extensively distributed throughout the CNS where, catalyzes the ACh synthesis (Oda 1999). Moreover, another study indicated that ChAT activity was significantly lower in nucleus accumbens from psychotic patients (Bird et al. 1997). In addition, postmortem study carried out by Freedman et al. (2000), has shown a reduction in the density of alpha7 neuronal nicotinic acetylcholine receptors in the hippocampus, striatum and prefrontal cortex of schizophrenic patients. These evidences indicate that schizophrenic patients have cholinergic abnormalities such as reduced acetylcholine in different brain structures (Bencherif et al. 2012). On the other hand, Nelson et al. (2002) showed that ketamine induced increase in cortical ACh release. These results corroborate with a research performed by Chatterjee et al. (2012) which shows that ketamine (acute and chronic) mediated increase in ACh concentration in the cerebral cortex and ketamine also induced increase in AChE activity in the cerebral cortex and hippocampus. This increase can be associated to a neuronal protection mechanism. Therefore, these findings indicate that activation of AChE in the cortical and hippocampal areas suggest an increased ACh turnover, further lends to idea that the ketamine induces cognitive deficit by cholinergic deregulation.
Another evidence that indicates a change in the cholinergic system in schizophrenic patients is the fact that schizophrenic patients smoke cigarettes at a much higher rate than in the general population (Sagud et al. 2009), since nicotine actives the cholinergic system by nicotinic receptors (Williams and Ganghi 2008; Winterer 2010). Data from literature have shown that consuming nicotine may ameliorate the negative symptoms of schizophrenia, leading to increased social interactions, pleasurable activities and improve of cognitive symptoms (Levin et al. 1996; Beratis et al. 2001; Combs and Advokat 2000; Poirier et al. 2002; DeLuca et al. 2004; Harris et al. 2004; Esterberg and Compton 2005). Therefore, nicotinic drugs may be an important therapeutic target to schizophrenia since that clinical studies indicated improve of attention consequently cognitive improve. However, it is important to observe the dosage and dosing schedule of these drugs. Moreover, a better understanding of the human biology of nicotinic receptor is essential for improving the successful clinical development of this promising target (AhnAllen 2012).
It is important to emphasize that few studies have investigated the cognitive role of rivastigmine in animal model relevant to schizophrenia. A preclinical study showed that AChE inhibitors such as physostigmine and donapezil improved the cognitive deficits induced by MK-801 in mice, an animal model of schizophrenia (Csernansky et al. 2005). Therefore, ours results become relevant to reinforce that AChE inhibitors such as rivastigmine, may be important to lessen the cognitive deficits produced by ketamine, another animal model of schizophrenia. Also, our results point to the need to assess AChE inhibitors in different animal model of schizophrenia since each animal model has different characteristics (specie of rodent, dose, time, effect and duration of effect). Besides the evaluation of behavioral effects of rivastigmine, we also assessed the effect of this AChE inhibitor drug in activity of acetylcholinesterase in the prefrontal cortex, hippocampus and striatum. The results of this work may have relevance for the behavioral and biochemical effects of cholinomimetic drugs in patients with schizophrenia.
In conclusion, this work indicates that rivastigmine might be effective in the reduction of cognitive deficit in different task (immediate memory, short term and long term memory) induced by chronic ketamine, an animal model of schizophrenia in rats. Moreover, rivastigmine reversed the increase of AChE activity in the prefrontal córtex, hippocampus and striatum of rats subjected to ketamine-induced animal model of schizophrenia. This effect may contribute to improve of cognitive impairment induced by rivastigmine on this animal model. However, this AChE inhibitor did not reverse the positive symptoms of schizophrenia (ketamine-induced hyperlocomotion), indicating specificity on cognitive function in the schizophrenia. Cholinergic system might be an important therapeutic target in the physiopathology of schizophrenia, mainly in the cognition, but additional studies should be carried.
Abbreviations
- ACh:
-
Acetylcholine
- AChE:
-
Acetylcholinesterase
- ChAT:
-
Choline acetyltransferase
- ChEI:
-
Acetylcholinesterase inhibitors
- CNS:
-
Central nervous system
- NMDA:
-
N-Methyl-D-aspartate
References
Aasen I, Kumari V, Sharma T (2005) Effects of rivastigmine on sustained attention in schizophrenia: an FMRI study. J Clin Psychopharmacol 25:311–317
Adell A, Jiménez-Sánchez L, López-Gil X, Romón T (2012) Is the acute NMDA receptor hypofunction a valid model of schizophrenia? Schizophr Bull 38(1):9–14
AhnAllen CG (2012) The role of the α7 nicotinic receptor in cognitive processing of persons with schizophrenia. Curr Opin Psychiatry 25(2):103–8. Review
Azevedo LM, Giera M, Lingeman H, Niessen WM (2011) Analysis of acetylcholinesterase inhibitors: bioanalysis, gradation and metabolism. Biomed Chromat 25:278–299
Barch MD, Ceaser A (2012) Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cognitive Sci 16:27–34
Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE (2008) The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacol 33(3):480–90
Barros R, Moreira P, Oliveira B (2005) Effect of social desirability on dietary intake estimated from a food questionnaire. Acta Med Port 18:241–247
Becker A, Grecksch G (2004) Ketamine-induced changes in rat behavior, a possible animal model of schizophrenia. Test of predictive validity. Prog Neuropsychopharmacol Biol Psychiatry 28:1267–1277
Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G (2003) Ketamine-induced changes in rat behavior: a possible animal model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 27:687–700
Bencherif M, Stachowiak MK, Kucinski AJ, Lippiello PM (2012) Alpha7 nicotinic cholinergic neuromodulation may reconcile multiple neurotransmitter hypotheses of schizophrenia. Med Hypotheses 78:594–600
Beratis S, Katrivanou A, Gourzis P (2001) Factors affecting smoking in schizophrenia. Compr Psychiatry 42:393–402
Bevilaqua LR, Rossato JI, Medina JH, Izquierdo I, Cammarota M (2003) Src kinase activity is required for avoidance memory formation and recall. Behav Pharmacol 14:649–652
Bird TD, Wijsman EM, Nochlin D, Leehey M, Sumi SM, Payami H, Poorkaj P, Nemens E, Rafkind M, Schellenberg GD (1997) Chromosome 17 and hereditary dementia: linkage studies in three non-Alzheimer families and kindreds with late-onset FAD. Neurology 48:949–954
Bressan RA, Pilowsky LS (2003) Glutamatergic hypothesis of schizophrenia. Rev Bras Psiquiatr 25:177–183
Canever L, Oliveira L, De Luca RD, Correa PTF, Fraga DB, Matos MP, Scaini G, Quevedo J, Streck EL, Zugno AI (2010) A rodent model of schizophrenia reveals increase in creatine kinase activity with associated behavior changes. Oxi Med Cel Long 6:421–427
Cardinal H, Madore F, St-Louis G, Bert JHE (2002) A predictive model for chronic allograft nephropathy. Nephropathy Transplant Procced 34:1810–1811
Chatterjee S, Khanna M (2011) Dimensional analysis of various rugae patterns in north Indian population Subset. Forensic Dent Sci 3:86–88
Chatterjee M, Verma R, Ganguly S, Palit G (2012) Neurochemical and molecular characterization of ketamineinduced experimental psychosis model in mice. Neuropharmacol 63(6):1161–71
Combs DR, Advokat C (2000) Antipsychotic medication and smoking prevalence in acutely hospitalized patients with chronic schizophrenia. Schizophr Res 46:129–137
Comim CM, Pereira JG, Steckert A, Petronilho F, Barichello T, Quevedo J, Dal-Pizzol F (2009) Rivastigmine reverses habituation memory impairment observed in sepsis survivor rats. Shock 32(3):270–271
Csernansky JG, Martin M, Shah R, Bertchume A, Colvin J, Dong H (2005) Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacol 30:2135–2143
Davis AJ, Holzbauer M, Sharnan DF (1975) Postnatal development of dopamine deamination in the striatum of the RAT. Br J Phlarmac 55:558–560
De Oliveira L, Spiazzi CM, Bortolin T, Canever L, Petronilho F, Mina FC, Dall Pizzol F, Quevedo J, Zugno AI (2009) Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog Neuropsychopharmacol Biol Psychiatry 33:1003–1008
De Oliveira L, Fraga DB, De Luca RD, Canever L, Ghedim FV, Matos MP, Streck EL, Quevedo J, Zugno AI (2011) Behavioral changes and mitochondrial dysfunction in a rat model of schizophrenia induced by ketamine. Metab Brain Dis 26:69–77
Deluca V, Wong AH, Muller DJ, Wong GW, Tyndale RF, Kennedy JL (2004) Evidence of association between smoking and alpha7 nicotinic receptor subunit gene in schizophrenia patients. Neuropsychopharmacol 29:1522–1526
Ellman GL, Courtney KD, Andres VJR, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Esterberg ML, Compton MT (2005) Smoking behavior in persons with a schizophrenia-spectrum disorder: a qualitative investigation of the transtheoretical model. Soc Sci Med 61:293–303
Fraga DB, Deroza PF, Ghedim FV, Streckert AV, De Luca RD, Silveiro A, Cipriano AL, Leffa DD, Borges GD, Quevedo J, Pinho RA, Andrade VM, Dal Pizzol F, Zugno AI (2011) Prenatal exposure to cigarette smoke causes persistent changes in the oxidative balance and in DNA structural integrity in rats sumitted to the animal model of schizophrenia. J Psychi Res 45:1497–1503
Freedman R, Adams CE, Leonard S (2000) The alpha7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J Chem Neuroanat 20:299–306
Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, Talpos J, Tricklebank M (2012) NMDA receptors, cognition and schizophrenia–testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology 62(3):1401–1412
Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerber G, Frredman R (2004) Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacol 29:1378–1385
Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch Gen Psychiatry in press
Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA (1986) Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 143:993–997
Izquierdo I, Medina JH (1998) On brain lesions, the milkman and Sigmunda
Jubelt LE, Barr RS, Goff DC, Logvinenko T, Weiss AP, Evins AE (2008) Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacol (Berl) 199(1):89–98. doi:10.1007/s00213-008-1133-8
Kane JM, Correll CU (2010) Pharmacologic treatment of schizophrenia. Dialogues Clin Neurosci 12(3):345–57. Review
Karson CN, Mrak RE, Husain MM, Griffin WS (1998) Decreased mesopontine choline acetyltransferase levels in schizophrenia. Correlations with cognitive functions. Mol Chem Neuropathol 29:181–191
Kumari V, Antonova E, Goyer MA, Ffytche D, Williamo SC, Sharma T (2006) A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int J Neuropsychopharmacol 10:463–477
Lenzi A, Maltinti E, Poggi E, Fabrizio L, Coli E (2003) Effects of rivastigmine on cognitive function and quality of life in patients with schizophrenia. Clin Neuropharmacol 26:317–321
Levin ED, Wilson W, Rose JE, Mcevoy J (1996) Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacol 15:429–436
Lima MJ, Tóth IV, Rangel AO (2005) A new approach for the sequential injection spectrophotometric determination of the total antioxidant activity. Talanta 15:207–213
Lodge DJ, Grace AA (2011) Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci 32:507–513
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Meltzer HY, Mcgurk SR (1999) The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25:233–255
Meyer U, Feldon J (2010) Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol 90:285–326
Mueser KT, Penn DL (2004) Meta-analysis examining the effects of social skills training on schizophrenia. Psychol Med 34:1365–1377
Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK (2010) Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther 128:419–432
Nelson CL, Burk JA, Bruno JP, Sarter M (2002) Effects of acute and repeated systemic administration of ketamine on prefrontal acetylcholine release and sustained attention performance in rats. Psychopharmacol (Berl) 161(2):168–79
Nieoullon A (2010) Acetylcholinesterase inhibitors in Alzheimer’s disease: further comments on their mechanisms of action and therapeutic consequences. Psychol Neuropsychiatr Vieil 8:123–131
Oda Y (1999) Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int 49:921–937
Olney JW, Newcomer JW, Farber NB (1999) NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 33:523–533
Perry W, Geyer MA, Braff DL (1999) Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry 56:277–281
Poirier MF, Canceil O, Baylé F, Millet B, Bourdel MC, Moatti C, Olié JP, Attar-Lévy D (2002) Prevalence of smoking in psychiatric patients. Prog Neuropsychopharmacol Biol Psychiatry 26:529–537
Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, Harvey PD, Davis KL (1998) Postmortem studies in schizophrenia. Schizophr Bull 24:325–341
Quevedo J, Moretto A, Colvero M, Roesler R, Ferreira MB (1997) The N-methyl-D-aspartate receptor blocker MK-801 prevents the facilitatory effects of naloxone and epinephrine on retention of inhibitory avoidance task in rats. Behav Pharmacol 8:471–474
Ribeiz SR, Bassitt DP, Arrais JÁ, Avila R, Steffens DC, Bottino CM (2010) Cholinesterase inhibitors as adjunctive therapy in patients with schizophrenia and schizoaffective disorder: a review and meta-analysis of the literature. CNS Drugs 24:303–317
Roesler R, Quevedo J, Schröder N (2003) NMDA receptors might be involved in the impairing effects of procyclidine on cognition. J Clin Psychopharmacol 23:666–668
Rogers TS, Kashima Y (1998) Nurses’ responses to people with schizophrenia. J Adv Nurs 27:195–203
Rogers A, Day JC, Williams B, Randall F, Wood P, Healy D, Bentall RP (1998) The meaning and management of neuroleptic medication: a study of patients with a diagnosis of schizophrenia. Soc Sci Med 47:1313–1323
Rösler S, Behr J, Richter E (1999) N-acetylcysteine treatment lowers 4-aminobiphenyl haemoglobin adduct levels in non-smokers. Eur J Cancer Prev 8:469–472
Sagud M, MihaL Jevic-Peles A, Muck-Seler D, Pivac N, Vuksan-Cusa B, Salgado JV, Hetem LA (2009) Experimental models of schizophrenia – a review. Rev Bras Psiquiatr 28:135–141
Sarter M, Lustig C, Taylor SF (2012) Cholinergic contributions to the cognitive symptoms of schizophrenia and the viability of cholinergic treatments. Neuropharmacol 62:1544–1553
Schultz SH, North SW, Shields CG (2007) Schizophrenia: a review. Am Fam Physician 75:1821–1829
Sesack SR, Carr DB (2002) Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav 77:513–517
Singh J, Kour K, Jayaram MB (2012) Acetylcholinesterase inhibitors for schizophrenia. Cochrane Database Syst Rev 1, CD007967
Tariot PN, Salzman C, Yeung PP, Pultz J, Rak IW (2000) Long-term use of Quetiapine in elderly patients with psychotic disorders. Clin Ther 22:1068–1084
Tzschentke TM, Schmidt WJ (2000) Differential effects of discrete subarea-specific lesions of the rat medial prefrontal cortex on amphetamine-and cocaine-induced behavioural sensitization. Cereb Cortex 10:488–498
Williams JM, Ganghi KK (2008) Use of caffeine and nicotine in people with schizophrenia. Curr Drug Abuse Rev 1:155–161
Winterer G (2010) Why do patients with schizophrenia smoke? Curr Opin Psychiatry 23:112–119
Wolf DH, Gerraty R, Satterthwaite TD, Loughead J, Campellone T, Elliott MA, Turetsky BI, Gur RE (2011) Striatal intrinsic reinforcement signals during recognition memory: relationship to response bias and dysregulation in schizophrenia. Front Behav Neurosci 5:81
Woolf NJ, Butcher LL (1986) Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull 16:603–637
Yakel JL (2013) Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease. Pflugers Arch in press
Financial Support
This Project was supported by L’Oreal, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Universidade do Extremo Sul Catarinense (UNESC) and FAPESC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zugno, A.I., Julião, R.F., Budni, J. et al. Rivastigmine reverses cognitive deficit and acetylcholinesterase activity induced by ketamine in an animal model of schizophrenia. Metab Brain Dis 28, 501–508 (2013). https://doi.org/10.1007/s11011-013-9417-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-013-9417-z