Abstract
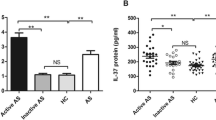
Ankylosing spondylitis (AS) is a chronic autoimmune disease. The purpose of this study was to investigate the levels of serum IL-36γ in AS patients and their association with AS. The study enrolled 131 subjects, including 45 with active AS, 46 with inactive AS, and 40 healthy controls (HCs). The basic clinical information of each participant was obtained through physical examination and relevant clinical medical records. Serum IL-36γ levels were detected through an enzyme-linked immunosorbent assay. Serum IL-36γ levels in the active AS group were significantly higher than those in the HC group (94.72 vs. 65.76 pg/mL, P = 0.0087). The serum IL-36γ concentration in the inactive AS group was increased as compared to that in the HC group (100.90 vs. 65.76 pg/mL, P = 0.0138). Correlation analysis indicated that serum IL-36γ was positively correlated with glutamyl transferase in the active AS group (P = 0.0172), while serum IL-36γ was positively correlated with uric acid in the inactive AS group (P = 0.0151). The area under the curve (AUC) for IL-36γ was 0.6824 (P = 0.0009), and the AUC for IL-36γ combined with the erythrocyte sedimentation rate and C-reactive protein levels was 0.8102 (P < 0.0001), according to receiver operating characteristic curve analysis. This study found that serum IL-36γ levels were elevated in AS patients and correlated with disease activity. Our results suggest that IL-36γ may be involved in the progression of AS disease and is a potential biomarker for AS.





Similar content being viewed by others
Data availability
Not applicable.
References
Zhu W, He X, Cheng K, Zhang L, Chen D, Wang X et al (2019) Ankylosing spondylitis: etiology, pathogenesis, and treatments. Bone Res 7:22
Brown MA, Kenna T, Wordsworth BP (2016) Genetics of ankylosing spondylitis–insights into pathogenesis. Nat Rev Rheumatol 12:81–91
Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ (2014) Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 53:650–657
Sieper J, Poddubnyy D, Miossec P (2019) The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol 15:747–757
McGonagle DG, McInnes IB, Kirkham BW, Sherlock J, Moots R (2019) The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann Rheum Dis 78:1167–1178
Gravallese EM, Schett G (2018) Effects of the IL-23-IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol 14:631–640
Mantovani A, Dinarello CA, Molgora M, Garlanda C (2019) Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 50:778–795
Bassoy EY, Towne JE, Gabay C (2018) Regulation and function of interleukin-36 cytokines. Immunol Rev 281:169–178
Elias M, Zhao S, Le HT, Wang J, Neurath MF, Neufert C et al (2021) IL-36 in chronic inflammation and fibrosis–bridging the gap? J Clin Invest. https://doi.org/10.1172/JCI144336
Chen WJ, Yu X, Yuan XR, Chen BJ, Cai N, Zeng S et al (2021) The role of IL-36 in the pathophysiological processes of autoimmune diseases. Front Pharmacol 12:727956
Chi HH, Hua KF, Lin YC, Chu CL, Hsieh CY, Hsu YJ et al (2017) IL-36 signaling facilitates activation of the NLRP3 Inflammasome and IL-23/IL-17 axis in renal inflammation and fibrosis. J Am Soc Nephrol 28:2022–2037
Pfaff CM, Marquardt Y, Fietkau K, Baron JM, Lüscher B (2017) The psoriasis-associated IL-17A induces and cooperates with IL-36 cytokines to control keratinocyte differentiation and function. Sci Rep 7:15631
Carrier Y, Ma HL, Ramon HE, Napierata L, Small C, O’Toole M et al (2011) Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J Invest Dermatol 131:2428–2437
Wang L, Wang FS, Gershwin ME (2015) Human autoimmune diseases: a comprehensive update. J Intern Med 278:369–395
Wang H, Li ZY, Jiang WX, Liao B, Zhai GT, Wang N et al (2018) The activation and function of IL-36γ in neutrophilic inflammation in chronic rhinosinusitis. J Allergy Clin Immunol 141:1646–1658
Li Q, Liu S, Li L, Ji X, Wang M, Zhou J (2019) Spinal IL-36γ/IL-36R participates in the maintenance of chronic inflammatory pain through astroglial JNK pathway. Glia 67:438–451
Zheng W, Hu X, Zou M, Hu N, Song W, Wang R et al (2023) Plasma IL-36α and IL-36γ as potential biomarkers in interstitial lung disease associated with rheumatoid arthritis: a pilot study in the Chinese population. Inflammation 46:285–296
Harusato A, Abo H, Ngo VL, Yi SW, Mitsutake K, Osuka S et al (2017) IL-36γ signaling controls the induced regulatory T cell-Th9 cell balance via NFκB activation and STAT transcription factors. Mucosal Immunol 10:1455–1467
Chowdhury K, Kumar U, Das S, Chaudhuri J, Kumar P, Kanjilal M et al (2018) Synovial IL-9 facilitates neutrophil survival, function and differentiation of Th17 cells in rheumatoid arthritis. Arthritis Res Ther 20:18
Jaber AS, Ad’hiah AH (2023) A novel signature of interleukins 36α, 37, 38, 39 and 40 in ankylosing spondylitis. Cytokine 162:156117
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Vanaki N, Aslani S, Jamshidi A, Mahmoudi M (2018) Role of innate immune system in the pathogenesis of ankylosing spondylitis. Biomed Pharmacother 105:130–143
Liao HT, Tsai CY (2023) Cytokines and regulatory T cells in ankylosing spondylitis. Bone Joint Res 12:133–137
Panchal NK, Prince SE (2023) Non-steroidal anti-inflammatory drugs (NSAIDs): A current insight into its molecular mechanism eliciting organ toxicities. Food Chem Toxicol 172:113598
Brennan PN, Dillon JF, Tapper EB (2022) Gamma-Glutamyl Transferase (γ-GT) - an old dog with new tricks? Liver Int 42:9–15
Boutet MA, Nerviani A, Pitzalis C (2019) IL-36, IL-37, and IL-38 cytokines in skin and joint inflammation: a comprehensive review of their therapeutic potential. Int J Mol Sci. https://doi.org/10.3390/ijms20061257
Kodali N, Blanchard I, Kunamneni S, Lebwohl MG (2023) Current management of generalized pustular psoriasis. Exp Dermatol 32:1204–1218
Funding
This work was supported by the National Natural Science Foundation of China (No. 81970735), Clinical Research and Application Project of Zhejiang Health Science and Technology Program (No. 2022KY1148), and Natural Science Foundation of Ningbo (No. 2022J233, No. 2021J080, No. 2021J246).
Author information
Authors and Affiliations
Contributions
SS wrote the main manuscript text and prepared Figs. 1–5. SS, WL, and YZ performed the experiment operation and data analysis. JP collected the clinical samples. YL and ML designed this study and provided funding support. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethics Committee of Ningbo No. 6 Hospital Affiliated to Ningbo University, which conducted according to the Declaration of Helsinki.
Consent to participate
All the participants in this study provided an informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, S., Lu, W., Zhou, Y. et al. Elevated serum IL-36γ levels in patients with ankylosing spondylitis and its association with disease activity. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04855-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04855-4