Abstract
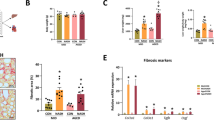
Pregnancy and lactation are important stages of fetal development. Therefore, this study investigated how different maternal diets offered during gestation and lactation periods affect adipose tissue inflammation and liver tissue oxidative stress of dams and their female offspring. Female BALB/c albino mice (60 days old) were randomized into three groups receiving a standard (CONT), hypercaloric (HD), or restricted (RD) diet during the pregnancy. After birth, female offspring weaned at 21 days were divided into two groups that received a standard or restricted diet (CONT/CONT, CONT/RD, RD/CONT, RD/RD, HD/CONT, and HD/RD) until 100 days old. Histological, oxidative parameters and inflammatory infiltrate of dams’ and offspring’s liver and adipose tissue were evaluated. HD dams presented non-alcoholic steatohepatitis (NASH) diagnosis and an increase in tumor necrosis factor-alpha (TNF-α) concentrations when compared to the RD and CONT dams, indicating a pro-inflammatory state. High concentrations of malondialdehyde (MDA) formation and catalase (CAT) activity in HD when compared to the CONT in the liver. SOD activity decreased in RD mice compared to CONT, and the SOD/CAT ratio was decreased in the RD and HD in comparison to the CONT. The maternal diet leads to an increase in SOD in RD/RD compared to HD/RD. RD-fed dams showed an increase in inflammatory infiltrates compared to CONT, evidencing changes caused by a restrictive diet. In the HD/CONT offspring, we verified an increase in inflammatory infiltrates in relation to the offspring fed a standard diet. In conclusion, HD, and RD, during pregnancy and lactation, altered the liver and adipose tissues of mothers. Furthermore, the maternal diet negatively impacts the offspring’s adipose tissue but does not cause liver damage in these animals in adult life.





Similar content being viewed by others
Data availability
Available upon request.
References
Schulz LC (2010) The Dutch hunger winter and the developmental origins of health and disease. Proc Natl Acad Sci USA 107:16757–16758. https://doi.org/10.1073/pnas.1012911107
Strakovsky RS, Pan YX (2012) In utero oxidative stress epigenetically programs antioxidant defense capacity and adulthood diseases. Antioxid Redox Signal 17(2):237–253. https://doi.org/10.1089/ars.2011.4372
Wadhwa PD, Buss C, Entringer S et al (2009) Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27:358–368. https://doi.org/10.1055/s-0029-1237424
Heindel JJ, Balbus J, Birnbaum L et al (2015) Developmental origins of health and disease: integrating environmental influences. Endocrinology 156(10):3416–3421. https://doi.org/10.1210/en.2015-1394
Dal Magro BM, Stone V, Klein CP et al (2020) Developmental programming: intrauterine caloric restriction promotes upregulation of mitochondrial sirtuin with mild effects on oxidative parameters in the ovaries and testes of offspring. Reprod Fertil Dev 32(8):763–773. https://doi.org/10.1071/rd19384
Stone V, Maurmann RM, Dal Magro BM et al (2021) Gestational caloric restriction with micronutrients supplementation does not delay development and promotes feeding behavior benefits. Nutr Neurosci 24(10):770–780. https://doi.org/10.1080/1028415x.2019.1676972
Toboła-Wróbel K, Pietryga M, Dydowicz P et al (2020) Association of oxidative stress on pregnancy. Oxid Med Cell Longev 2020(1):6398520
Gawlińska K, Gawliński D, Filip M et al (2021) Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr Rev 79(6):709–725. https://doi.org/10.1093/nutrit/nuaa020
Zinkhan EK, Yu B, Callaway CW et al (2018) Intrauterine growth restriction combined with a maternal high-fat diet increased adiposity and serum corticosterone levels in adult rat offspring. J Dev Orig Health Dis 9(3):315–328. https://doi.org/10.1017/s2040174418000016
Yki-Järvinen H (2014) Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2(11):901–910. https://doi.org/10.1016/s2213-8587(14)70032-4
Gardner DS, Tingey K, Van Bon BW et al (2005) Programming of glucose-insulin metabolism in adult sheep after maternal undernutrition. Am J Physiol Regul Integr Comp Physiol 289(4):R947–R954. https://doi.org/10.1152/ajpregu.00120.2005
van der Heijden RA, Sheedfar F, Morrison MC et al (2015) High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging 7(4):256
Masarone M, Rosato V, Dallio M et al (2018) Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev 2018(2018):9547613. https://doi.org/10.1155/2018/9547613
Rolo AP, Teodoro JS, Palmeira CM (2012) Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med 52:59–69. https://doi.org/10.1016/j.freeradbiomed.2011.10.003
Yoon HJ, Cha BS (2014) Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J Hepatol 6:800–811. https://doi.org/10.4254/wjh.v6.i11.800
Colman RJ, Beasley TM, Kemnitz JW et al (2014) Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 5:3557. https://doi.org/10.1038/ncomms4557
Lee C, Longo V (2016) Dietary restriction with and without caloric restriction for healthy aging. F1000Res. https://doi.org/10.12688/f1000research.7136.1
Fisch J, Feistauer V, de Moura AC et al (2019) Maternal feeding associated to post-weaning diet affects metabolic and behavioral parameters in female offspring. Physiol Behav 204:162–167. https://doi.org/10.1016/j.physbeh.2019.02.026
Kleiner DE, Brunt EM, Van Natta M et al (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41(6):1313–1321. https://doi.org/10.1002/hep.20701
Azambuja JH, da Silveira EF, de Carvalho TR et al (2017) Glioma sensitive or chemoresistant to temozolomide differentially modulate macrophage protumor activities. Biochim Biophys Acta Gen Subj 1861(11 Pt A):2652–2662. https://doi.org/10.1016/j.bbagen.2017.07.007
Luft JG, Steffens L, Morás AM et al (2019) Rosmarinic acid improves oxidative stress parameters and mitochondrial respiratory chain activity following 4-aminopyridine and picrotoxin-induced seizure in mice. Naunyn Schmiedebergs Arch Pharmacol 392(11):1347–1358. https://doi.org/10.1007/s00210-019-01675-6
Salgo MG, Pryor WA (1996) Trolox inhibits Peroxynitrite-mediated oxidative stress and apoptosis in rat thymocytes. Arch Biochem Biophys 333(2):482–488. https://doi.org/10.1006/abbi.1996.0418
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Erlbaum, Hillsdale
Gauthier MF, de Andrade AA, Fisch J (2022) Dietary interventions in mice affect oxidative stress and gene expression of the Prlr and Esr1 in the adipose tissue and hypothalamus of dams and their offspring. J Physiol Biochem 1:1–2
Kucera O, Cervinkova Z (2014) Experimental models of non-alcoholic fatty liver disease in rats. World J Gastroenterol 20(26):8364–8376
Taylor PD, Poston L (2007) Developmental programming of obesity in mammals. Exp Physiol 92(2):287–298. https://doi.org/10.1113/expphysiol.2005.032854
Fleeman TL, Cappon GD, Chapin RE et al (2005) The effects of feed restriction during organogenesis on embryo-fetal development in the rat. Birth Defects Res B Dev Reprod Toxicol 74(5):442–449. https://doi.org/10.1002/bdrb.20056
Fowden AL, Forhead AJ (2004) Endocrine mechanisms of intrauterine programming. Reproduction 127(5):515–526. https://doi.org/10.1530/rep.1.00033
Noeman SA, Hamooda HE, Baalash AA (2011) Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr 3(1):1–8. https://doi.org/10.1186/1758-5996-3-17
Mohammadi M, Ghaznavi R, Keyhanmanesh R et al (2014) Caloric restriction prevents lead-induced oxidative stress and inflammation in rat liver. Sci World J 2014:821524. https://doi.org/10.1155/2014/821524
Wankhade UD, Zhong Y, Kang P et al (2017) Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS One 12(4):e0175675. https://doi.org/10.1371/journal.pone.0175675
Muñoz A, Costa M (2013) Nutritionally mediated oxidative stress and inflammation. Oxid Med Cell Longev 2013:610950. https://doi.org/10.1155/2013/610950
Bloomfield FH, Oliver MH, Giannoulias CD et al (2003) Brief undernutrition in late-gestation sheep programs the hypothalamic-pituitary-adrenal axis in adult offspring. Endocrinology 144(7):2933–2940. https://doi.org/10.1210/en.2003-0189
Umekawa T, Sugiyama T, Du Q et al (2015) A maternal mouse diet with moderately high-fat levels does not lead to maternal obesity but causes mesenteric adipose tissue dysfunction in male offspring. J Nutr Biochem 26(3):259–266. https://doi.org/10.1016/j.jnutbio.2014.10.012
Dos Santos LS, de Matos RJ, Cordeiro GD et al (2022) Perinatal and post-weaning exposure to an obesogenic diet promotes greater expression of nuclear factor-κB and tumor necrosis factor-α in white adipose tissue and hypothalamus of adult rats. Nutr Neurosci 25(3):502–510. https://doi.org/10.1080/1028415x.2020.1764291
López-Lluch G, Navas P (2016) Calorie restriction as an intervention in ageing. J Physiol 594(8):2043–2060
Pifferi F, Aujard F (2019) Caloric restriction, longevity and aging: recent contributions from human and non-human primate studies. Prog Neuropsychopharmacol Biol Psychiatry 95:109702. https://doi.org/10.1016/j.pnpbp.2019.109702
Fabbiano S, Suárez-Zamorano N, Rigo D et al (2016) Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab 24(3):434–446. https://doi.org/10.1016/j.cmet.2016.07.023
Miller KN, Burhans MS, Clark JP et al (2017) Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell 16(3):497–507. https://doi.org/10.1111/acel.12575
Corrales P, Vidal-Puig A, Medina-Gómez G (2018) PPARs and metabolic disorders associated with challenged adipose tissue plasticity. Int J Mol Sci 19(7):2124
Acknowledgements
Not applicable.
Funding
This work was supported by CAPES/Brazil and PROAP/UFCSPA.
Author information
Authors and Affiliations
Contributions
JF, AGB and MG conceived and designed the study. JF, ACM, VF, LSR, AMM, and POS performed the experiment. RPG, AGB and MG analyzed and interpreted all the data. All authors were involved in drafting and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethical approval
All animals were treated with humane conditions, following the institutional guidelines for the care and use of animals.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fisch, J., de Moura, A.C., Feistauer, V. et al. Effects of different maternal diets on adipose tissue inflammation and liver tissue oxidative stress in dams and their female offspring. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04791-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04791-3