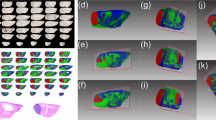
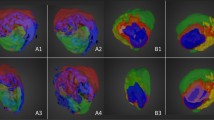
Abstract
Breast cancer brain metastasis (BCBM) has an incidence of 10–30%. It is incurable and the biological mechanisms that promote its progression remain largely undefined. Consequently, to gain insights into BCBM processes, we have developed a spontaneous mouse model of BCBM and in this study found a 20% penetrance of macro-metastatic brain lesion formation. Considering that lipid metabolism is indispensable to metastatic progression, our goal was the mapping of lipid distributions throughout the metastatic regions of the brain. Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) of lipids revealed that, relative to surrounding brain tissue, seven long-chain (13–21 carbons long) fatty acylcarnitines, as well as two phosphatidylcholines, two phosphatidylinositols two diacylglycerols, a long-chain phosphatidylethanolamine, and a long-chain sphingomyelin were highly concentrated in the metastatic brain lesion In broad terms, lipids known to be enriched in brain tissues, such as very long-chain (≥ 22 carbons in length) polyunsaturated fatty acid of phosphatidylcholines, phosphatidylethanolamine, sphingomyelins, sulfatides, phosphatidylinositol phosphates, and galactosylceramides, were not found or only found in trace amounts in the metastatic lesion and instead consistently detected in surrounding brain tissues. The data, from this mouse model, highlights an accumulation of fatty acylcarnitines as possible biological makers of a chaotic inefficient vasculature within the metastasis, resulting in relatively inadequate blood flow and disruption of fatty acid β-oxidation due to ischemia/hypoxia.






Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Bryan S, Witzel I, Borgmann K, Oliveira-Ferrer L (2021) Molecular mechanisms associated with brain metastases in HER2-positive and triple negative breast cancers. Cancers (Basel). https://doi.org/10.3390/cancers13164137
Cancer.Net https://www.cancer.net/cancer-types/breast-cancer/statistics.
Guo R, Chen Y, Borgard H, Jijiwa M, Nasu M, He M, Deng Y (2020) The function and mechanism of lipid molecules and their roles in the diagnosis and prognosis of breast cancer. Molecules. https://doi.org/10.3390/molecules25204864
Valiente M, Van Swearingen AED, Anders CK, Bairoch A, Boire A, Bos PD, Cittelly DM, Erez N, Ferraro GB, Fukumura D, Gril B, Herlyn M, Holmen SL, Jain RK, Joyce JA, Lorger M, Massague J, Neman J, Sibson NR, Steeg PS, Thorsen F, Young LS, Vareslija D, Vultur A, Weis-Garcia F, Winkler F (2020) Brain metastasis cell lines panel: a public resource of organotropic cell lines. Cancer Res 80:4314–4323. https://doi.org/10.1158/0008-5472.CAN-20-0291
Shah N, Mohammad AS, Saralkar P, Sprowls SA, Vickers SD, John D, Tallman RM, Lucke-Wold BP, Jarrell KE, Pinti M, Nolan RL, Lockman PR (2018) Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol Res 132:47–68. https://doi.org/10.1016/j.phrs.2018.03.021
Winnard PT Jr, Vesuna F, Muthukumar S, Raman V (2020) Divergent organ-specific isogenic metastatic cell lines identified using multi-omics exhibit differential drug sensitivity. PLoS One 15:e0242384. https://doi.org/10.1371/journal.pone.0242384
Winnard PT Jr, Zhang C, Vesuna F, Kang JW, Garry J, Dasari RR, Barman I, Raman V (2017) Organ-specific isogenic metastatic breast cancer cell lines exhibit distinct Raman spectral signatures and metabolomes. Oncotarget 8:20266–20287. https://doi.org/10.18632/oncotarget.14865
Zhang C, Winnard PT Jr, Dasari S, Kominsky SL, Doucet M, Jayaraman S, Raman V, Barman I (2018) Label-free Raman spectroscopy provides early determination and precise localization of breast cancer-colonized bone alterations. Chem Sci 9:743–753. https://doi.org/10.1039/c7sc02905e
Sun C, Wang F, Zhang Y, Yu J, Wang X (2020) Mass spectrometry imaging-based metabolomics to visualize the spatially resolved reprogramming of carnitine metabolism in breast cancer. Theranostics 10:7070–7082. https://doi.org/10.7150/thno.45543
Jackson SN, Wang HY, Woods AS (2005) Direct profiling of lipid distribution in brain tissue using MALDI-TOFMS. Anal Chem 77:4523–4527. https://doi.org/10.1021/ac050276v
Jackson SN, Wang HY, Woods AS, Ugarov M, Egan T, Schultz JA (2005) Direct tissue analysis of phospholipids in rat brain using MALDI-TOFMS and MALDI-ion mobility-TOFMS. J Am Soc Mass Spectrom 16:133–138. https://doi.org/10.1016/j.jasms.2004.10.002
Wang HY, Jackson SN, Post J, Woods AS (2008) A minimalist approach to MALDI imaging of glycerophospholipids and sphingolipids in rat brain sections. Int J Mass Spectrom 278:143–149. https://doi.org/10.1016/j.ijms.2008.04.005
Colsch B, Jackson SN, Dutta S, Woods AS (2011) Molecular microscopy of brain gangliosides: illustrating their distribution in hippocampal cell layers. ACS Chem Neurosci 2:213–222. https://doi.org/10.1021/cn100096h
Muller L, Baldwin K, Barbacci DC, Jackson SN, Roux A, Balaban CD, Brinson BE, McCully MI, Lewis EK, Schultz JA, Woods AS (2017) Laser desorption/ionization mass spectrometric imaging of endogenous lipids from rat brain tissue implanted with silver nanoparticles. J Am Soc Mass Spectrom 28:1716–1728. https://doi.org/10.1007/s13361-017-1665-4
Calvano CD, Ventura G, Palmisano F, Cataldi TR (2016) 4-Chloro-alpha-cyanocinnamic acid is an efficient soft matrix for cyanocobalamin detection in foodstuffs by matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS). J Mass Spectrom 51:841–848. https://doi.org/10.1002/jms.3817
Chughtai K, Jiang L, Greenwood TR, Glunde K, Heeren RM (2013) Mass spectrometry images acylcarnitines, phosphatidylcholines, and sphingomyelin in MDA-MB-231 breast tumor models. J Lipid Res 54:333–344. https://doi.org/10.1194/jlr.M027961
Medes G, Thomas A, Weinhouse S (1953) Metabolism of neoplastic tissue. IV: a study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res 13:27–29
Weinhouse S, Millington RH, Wenner CE (1951) Metabolism of neoplastic tissue. I.: the oxiation of carbohydrate and fatty acids in transplanted tumors. Cancer Res 11:845–850
Emmelot P, Bos CJ (1955) Factors influencing the fatty acid oxidation of tumor mitochondria with special reference to changes in spontaneous mouse hepatomas. Experientia 11:353–354. https://doi.org/10.1007/BF02159918
Bhadwal P, Dahiya D, Shinde D, Vaiphei K, Math RGH, Randhawa V, Agnihotri N (2020) LC-HRMS based approach to identify novel sphingolipid biomarkers in breast cancer patients. Sci Rep 10:4668. https://doi.org/10.1038/s41598-020-61283-w
Marino N, German R, Rao X, Simpson E, Liu S, Wan J, Liu Y, Sandusky G, Jacobsen M, Stoval M, Cao S, Storniolo AMV (2020) Upregulation of lipid metabolism genes in the breast prior to cancer diagnosis. NPJ Breast Cancer 6:50. https://doi.org/10.1038/s41523-020-00191-8
Monaco ME (2017) Fatty acid metabolism in breast cancer subtypes. Oncotarget 8:29487–29500. https://doi.org/10.18632/oncotarget.15494
Attane C, Muller C (2020) Drilling for oil: tumor-surrounding adipocytes fueling cancer. Trends Cancer 6:593–604. https://doi.org/10.1016/j.trecan.2020.03.001
De Oliveira MP, Liesa M (2020) The role of mitochondrial fat oxidation in cancer cell proliferation and survival. Cells. https://doi.org/10.3390/cells9122600
Koundouros N, Poulogiannis G (2020) Reprogramming of fatty acid metabolism in cancer. Br J Cancer 122:4–22. https://doi.org/10.1038/s41416-019-0650-z
Porporato PE, Payen VL, Perez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T, Dhup S, Tardy M, Vazeille T, Bouzin C, Feron O, Michiels C, Gallez B, Sonveaux P (2014) A mitochondrial switch promotes tumor metastasis. Cell Rep 8:754–766. https://doi.org/10.1016/j.celrep.2014.06.043
Ferraro GB, Ali A, Luengo A, Kodack DP, Deik A, Abbott KL, Bezwada D, Blanc L, Prideaux B, Jin X, Possada JM, Chen J, Chin CR, Amoozgar Z, Ferreira R, Chen I, Naxerova K, Ng C, Westermark AM, Duquette M, Roberge S, Lindeman NI, Lyssiotis CA, Nielsen J, Housman DE, Duda DG, Brachtel E, Golub TR, Cantley LC, Asara JM, Davidson SM, Fukumura D, Dartois VA, Clish CB, Jain RK, Vander Heiden MG (2021) Fatty acid synthesis is required for breast cancer brain metastasis. Nat Cancer 2:414–428. https://doi.org/10.1038/s43018-021-00183-y
Mao X, He J, Li T, Lu Z, Sun J, Meng Y, Abliz Z, Chen J (2016) Application of imaging mass spectrometry for the molecular diagnosis of human breast tumors. Sci Rep 6:21043. https://doi.org/10.1038/srep21043
Paine MRL, Liu J, Huang D, Ellis SR, Trede D, Kobarg JH, Heeren RMA, Fernandez FM, MacDonald TJ (2019) Three-dimensional mass spectrometry imaging identifies lipid markers of medulloblastoma metastasis. Sci Rep 9:2205. https://doi.org/10.1038/s41598-018-38257-0
Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang W, Wang XY, Fang X (2018) Fatty acid oxidation: an emerging facet of metabolic transformation in cancer. Cancer Lett 435:92–100. https://doi.org/10.1016/j.canlet.2018.08.006
Melone MAB, Valentino A, Margarucci S, Galderisi U, Giordano A, Peluso G (2018) The carnitine system and cancer metabolic plasticity. Cell Death Dis 9:228. https://doi.org/10.1038/s41419-018-0313-7
Han S, Wei R, Zhang X, Jiang N, Fan M, Huang JH, Xie B, Zhang L, Miao W, Butler AC, Coleman MA, Vaughan AT, Wang Y, Chen HW, Liu J, Li JJ (2019) CPT1A/2-Mediated FAO enhancement-a metabolic target in radioresistant breast cancer. Front Oncol 9:1201. https://doi.org/10.3389/fonc.2019.01201
Wang J, Xiang H, Lu Y, Wu T, Ji G (2021) The role and therapeutic implication of CPTs in fatty acid oxidation and cancers progression. Am J Cancer Res 11:2477–2494
Yaligar J, Teoh WW, Othman R, Verma SK, Phang BH, Lee SS, Wang WW, Toh HC, Gopalan V, Sabapathy K, Velan SS (2016) Longitudinal metabolic imaging of hepatocellular carcinoma in transgenic mouse models identifies acylcarnitine as a potential biomarker for early detection. Sci Rep 6:20299. https://doi.org/10.1038/srep20299
Al-Bakheit A, Traka M, Saha S, Mithen R, Melchini A (2016) Accumulation of palmitoylcarnitine and its effect on pro-inflammatory pathways and calcium influx in prostate cancer. Prostate 76:1326–1337. https://doi.org/10.1002/pros.23222
Zhou L, Wang Q, Yin P, Xing W, Wu Z, Chen S, Lu X, Zhang Y, Lin X, Xu G (2012) Serum metabolomics reveals the deregulation of fatty acids metabolism in hepatocellular carcinoma and chronic liver diseases. Anal Bioanal Chem 403:203–213. https://doi.org/10.1007/s00216-012-5782-4
Dambrova M, Zuurbier CJ, Borutaite V, Liepinsh E, Makrecka-Kuka M (2021) Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic Biol Med 165:24–37. https://doi.org/10.1016/j.freeradbiomed.2021.01.036
Ford DA, Han X, Horner CC, Gross RW (1996) Accumulation of unsaturated acylcarnitine molecular species during acute myocardial ischemia: metabolic compartmentalization of products of fatty acyl chain elongation in the acylcarnitine pool. Biochemistry 35:7903–7909. https://doi.org/10.1021/bi960552n
Liepinsh E, Makrecka-Kuka M, Volska K, Kuka J, Makarova E, Antone U, Sevostjanovs E, Vilskersts R, Strods A, Tars K, Dambrova M (2016) Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. Biochem J 473:1191–1202. https://doi.org/10.1042/BCJ20160164
Shug AL, Thomsen JH, Folts JD, Bittar N, Klein MI, Koke JR, Huth PJ (1978) Changes in tissue levels of carnitine and other metabolites during myocardial ischemia and anoxia. Arch Biochem Biophys 187:25–33. https://doi.org/10.1016/0003-9861(78)90003-6
Whitmer JT, Idell-Wenger JA, Rovetto MJ, Neely JR (1978) Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem 253:4305–4309
Chen CT, Bazinet RP (2015) beta-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot Essent Fatty Acids 92:33–40. https://doi.org/10.1016/j.plefa.2014.05.007
DeMar JC Jr, Lee HJ, Ma K, Chang L, Bell JM, Rapoport SI, Bazinet RP (2006) Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta 1761:1050–1059. https://doi.org/10.1016/j.bbalip.2006.06.006
Demar JC Jr, Ma K, Chang L, Bell JM, Rapoport SI (2005) alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. J Neurochem 94:1063–1076. https://doi.org/10.1111/j.1471-4159.2005.03258.x
Jenkins B, de Schryver E, Van Veldhoven PP, Koulman A (2017) Peroxisomal 2-hydroxyacyl-CoA lyase is involved in endogenous biosynthesis of heptadecanoic acid. Molecules. https://doi.org/10.3390/molecules22101718
Violante S, Achetib N, van Roermund CWT, Hagen J, Dodatko T, Vaz FM, Waterham HR, Chen H, Baes M, Yu C, Argmann CA, Houten SM (2019) Peroxisomes can oxidize medium- and long-chain fatty acids through a pathway involving ABCD3 and HSD17B4. FASEB J 33:4355–4364. https://doi.org/10.1096/fj.201801498R
Wanders RJA, Vaz FM, Waterham HR, Ferdinandusse S (2020) Fatty acid oxidation in peroxisomes: enzymology, metabolic crosstalk with other organelles and peroxisomal disorders. Adv Exp Med Biol 1299:55–70. https://doi.org/10.1007/978-3-030-60204-8_5
Babak MV, Zalutsky MR, Balyasnikova IV (2020) Heterogeneity and vascular permeability of breast cancer brain metastases. Cancer Lett 489:174–181. https://doi.org/10.1016/j.canlet.2020.06.012
Nalecz KA, Miecz D, Berezowski V, Cecchelli R (2004) Carnitine: transport and physiological functions in the brain. Mol Aspects Med 25:551–567. https://doi.org/10.1016/j.mam.2004.06.001
Yoon DW, Kwon HN, Jin X, Kim JK, Lee SK, Park S, Yun CH, Shin C (2019) Untargeted metabolomics analysis of rat hippocampus subjected to sleep fragmentation. Brain Res Bull 153:74–83. https://doi.org/10.1016/j.brainresbull.2019.08.008
Griner EM, Kazanietz MG (2007) Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer 7:281–294. https://doi.org/10.1038/nrc2110
Kawashima M, Iwamoto N, Kawaguchi-Sakita N, Sugimoto M, Ueno T, Mikami Y, Terasawa K, Sato TA, Tanaka K, Shimizu K, Toi M (2013) High-resolution imaging mass spectrometry reveals detailed spatial distribution of phosphatidylinositols in human breast cancer. Cancer Sci 104:1372–1379. https://doi.org/10.1111/cas.12229
Funding
This research was supported in part by the Intramural Research Program of the National Institute on Drug Abuse, NIH. Venu Raman acknowledges support from Grant 1RO1CA207208.
Author information
Authors and Affiliations
Contributions
AR and MHVV designed and carried out the cell culture and animal experiments. PW generated the cell line, contributed to the writing and editing of main manuscript and contributed to the preparation of the figures. LM, SJ, BH, AR and AW designed and carried out the MALDI-MSI analysis. AR and AW contributed to the preparation of the figures and the writing and editing of the main manuscript. AW, VR designed the study and VR edited the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roux, A., Winnard, P.T., Van Voss, M.H. et al. MALDI-MSI of lipids in a model of breast cancer brain metastasis provides a surrogate measure of ischemia/hypoxia. Mol Cell Biochem 478, 2567–2580 (2023). https://doi.org/10.1007/s11010-023-04685-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04685-4