Abstract
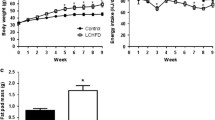
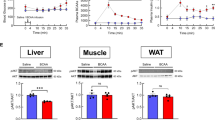
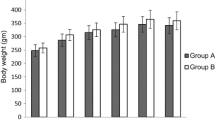
Obesity is a relevant health public issue and is the main factor for glucose metabolism dysregulation and diabetes progression; however, the differential role of a high-fat diet or high sugar diet consumption on glucose metabolism and insulin processing is not well understood and has been scarcely described. Our research aimed to analyze the effects of chronic consumption of both high sucrose and high-fat diets on glucose and insulin metabolism regulation. Wistar rats were fed with high-sugar or high-fat diets for 12 months; after that, fasting glucose and insulin levels were measured along with a glucose tolerance test (GTT). Proteins related to insulin synthesis and secretion were quantified in pancreas homogenates, whereas islets were isolated to analyze ROS generation and size measurement. Our results show that both diets induce metabolic syndrome, linked with central obesity, hyperglycemia, and insulin resistance. We observed alterations in the expression of proteins related with insulin synthesis and secretion, along with diminution of Langerhans islets size. Interestingly, the severity and number of alterations were more evident in the high-sugar diet than in the high-fat diet group. In conclusion, obesity and glucose metabolism dysregulation induced by carbohydrate consumption, led to worst outcomes than high-fat diet.



Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
De Tursi RL, Vázquez Tarragón A, Vázquez Prado A, Sáenz Tormo G, Gambau Puchol V (2013) Relationship of oxidative stress and weight loss obtained in morbidly obese patients by bariatric surgery with the duodenal switch technique. Nutr Hosp 28(3):1085–1092
Higdon JV, Frei B (2003) Obesity and oxidative stress. Arterioscler Thromb Vasc Biol 23:365–367
Bensellam M, Jonas JC, Laybutt DR (2018) Mechanisms of β-cell dedifferentiation in diabetes: recent findings and future research directions. J Endocrinol 236(2):R109–R143
Burgeiro A, Cerqueira MG, Varela-Rodríguez BM, Nunes S, Neto P, Pereira FC, Reis F, Carvalho E (2017) Glucose and lipid dysmetabolism in a rat model of prediabetes induced by a high-sucrose diet. Nutrients 9(6):638
Gómez-Crisóstomo NP, De la Cruz Hernández EN, Méndez Méndez ER, Hernández-Landero MF, Camacho Liévano JU, Martínez-Abundis E (2018) Differential effect of high-fat, high-sucrose and combined high fat/high-sucrose diets consumption on fat accumulation, serum leptin and cardiac hypertrophy in rats. Arch Physiol Biochem 126(3):258–263
Matos Ferreira AV, Menezes-Garcia Z, Baeta Viana J, Guilhen Mario E, Botion LM (2014) Distinct metabolic pathways trigger adipocyte fat accumulation induced by high-carbohydrate and high-fat diets. Nutrition 30(2):1138–1143
Sergi D, Naumovski N, Heilbronn LK, Abeywardena M, O’Callaghan N, Lionetti L, Luscombe-Marsh N (2019) Mitochondrial (Dys)function and Insulin resistance: from pathophysiological molecular mechanisms to the impact of diet. Front Physiol 10:532
Pepin É, Al-Mass A, Attané C, Zhang K, Lamontagne J, Lussier R, Madiraju SR, Joly E, Ruderman NB, Sladek R et al (2016) Pancreatic β-cell dysfunction in diet-induced obese mice: roles of amp-kinase, protein kinase Cε, mitochondrial and cholesterol metabolism, and alterations in gene expression. PLoS ONE 11(4):e0153017
Cruz Hernández JH, Rosado Lomán WN, Gómez-Crisóstomo NP, De la Cruz-Hernández EN, Guzmán García LM, Gómez Gómez M, Hernández Del Ángel NA, Aguilar Gamas CF, Cruz Hernández VS, Martínez-Abundis E (2023) High sugar but not high fat diet consumption induces hepatic metabolic disruption and up-regulation of mitochondrial fission-associated protein Drp 1 in a model of moderate obesity. Arch Physiol Biochem 129(1):233–240
Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, Fernandes AA, Cicogna AC, Novelli Filho JL (2007) Anthropometrical parameters and markers of obesity in rats. Lab Anim 41(1):111–119
Ram H, Kumar P, Purohit A, Kashyap P, Kumar S, Kumar S, Singh G, Alqarawi AA, Hashem A, Abd-Allah EF, Al-Arjani AF, Singh BP (2021) Improvements in HOMA indices and pancreatic endocrinal tissues in type 2-diabetic rats by DPP-4 inhibition and antioxidant potential of an ethanol fruit extract of Withania coagulans. Nutr Metab (Lond) 18(1):43
Canul-Medina G, Riverón-Negrete L, Pastén-Hidalgo K, Morales-Castillo P, García-Vázquez F, Fernandez-Mejia C (2021) Maternal adaptations of pancreatic islets and glucose metabolism after lactation. J Endocrinol 248(1):1–15
Martinez-Abundis E, Rajapurohitam V, Haist J, Gan X, Karmazyn M (2012) The Obesity-Related peptide leptin sensitizes cardiac mitochondria to calcium-induced permeability transition pore opening and apoptosis. PLoS ONE 7(7):e41612
Baines CP, Gutiérrez-Aguilar M (2018) The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium 73:121–130
Schwarz JM, Noworolski SM, Dyachenko WMJ, A, Prior JL, Weinberg ME, Herraiz LA, Tai VW, et al (2015) Effects of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J Clin Endocrinol Metab 100(6):2434–2442
Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, Yancy WS, Phinney SD (2007) Low-carbohydrate nutrition and metabolism. Am J Clin Nutr 86:276–284
Lirio LM, Forechi L, Zanardo TC, Batista HM, Meira EF, Nogueira BV, Mill JG, Baldo MP (2016) Chronic fructose ingestion accelerates non-alcoholic fatty liver disease in the presence of essential hypertension. J Diabetes Complicat 30:85–92
Luna López V, López Medina JA, Vázquez Gutiérrez M, Fernández Soto ML (2014) Carbohydrates: update on their role in diabetes mellitus and metabolic disease. Nutr Hosp 30(5):1020–1031
Galgani J, Ravussin E (2008) Energy metabolism, fuel selection, and body weight regulation. Intl J Obes (Lond) 32:S109–S119
Hamidi Shishavan M, Henning RH, van Buiten A, Goris M, Deelman LE, Buikema H (2017) Metformin improves endothelial function and reduces blood pressure in diabetic spontaneously hypertensive rats independent from glycemia control: comparison to vildagliptin. Sci Rep 7(1):10975
Ferland DJ, Flood ED, Garver H, Yeh ST, Riney S, Mullick AE, Fink GD, Watts SW (2019) Different blood pressure responses in hypertensive rats following chemerin mRNA inhibition in dietary high fat compared to dietary high-salt conditions. Physiol Genom 51(11):553–561
Robles NR, Macias JF (2015) Hypertension in the elderly. Cardiovasc Hematol Agents Med Chem 12(3):136–145
Pouliot MC, Dépres JP, Lemieux S, Moorjani S, Tremblay A, Nadeau A (1994) Wiast circunference and abdominal sagital diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 73(1):46–468
Schmidt MI, Watson RL, Duncan BB, Metcalf P, Brancati FL, Sharrett AR (1996) Clustering of dyslipidemia, hyperuricemia, diabetes, and blood pressure and its association with fasting insulin and central overall obesity in a general population. Metabolism 45:699–706
Popkin BM, Hawkes C (2016) Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol 4:174–186
Malik VS, Hu FB (2019) Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients 11:1840
Tappy L, Lê KA (2010) Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90(1):23–46
Schaefer EJ, Gleason JA, Dansinger ML (2009) Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J Nutr 139:1257–1262
Pan Y, Wang B, Zheng J, Xiong R, Fan Z, Ye Y, Zhang S, Li Q, Gong F, Wu C et al (2019) Pancreatic fibroblast growth factor 21 protects against type 2 diabetes in mice by promoting insulin expression and secretion in a PI3K/Akt signaling-dependent manner. J Cell Mol Med 23(2):1059–1071
Fu Z, Gilbert ER, Liu D (2013) Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diab Rev 9(1):25–53
Vela-Guajardo JE, Garza-González S, García N (2021) Glucolipotoxicity-induced oxidative stress is related to mitochondrial dysfunction and apoptosis of pancreatic β-cell. Curr Diab Rev 17(5):e031120187541
Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, Zhou T (2019) Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int J Mol Sci 20(18):4472
Acknowledgements
Authors want to thank Xadeni Burgos Gamez and Dr. Cristina Fernández-Mejía for their technical support for isolation of islets.
Funding
Márquez Álvarez CM was supported by a fellowship from “Consejo Nacional de Ciencia y Tecnología, México” (CONACYT) as part of “Programa de Doctorado en Ciencias Biomédicas, DACS-UJAT”, CVU# 909241. This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
CMMA, NPG-C, ENDC-H and EM-A: contributed to the conception and design of the work. CMMA, NPG-C, CFA-G and EM-A: participate in perform the experiments, the acquisition, analysis, and interpretation of data. CZ and EM-A: were a major contributors in writing the manuscript, drafted the work and revised it. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Rats were purchased from the Production, Care and Animal Experimentation Unit (UPCEA), Juarez Autonomous University of Tabasco. Experiments were designed following the Mexican regulations for use and research in animals (NOM-062-ZOO-1999) and the “Three R’s”: replacement, reduction, and refinement in research with animals; additionally, the protocol was subjected to approbation by the Institutional Committee for Ethics in Research (Protocol #0423).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de María Márquez Álvarez, C., Gómez-Crisóstomo, N.P., De la Cruz-Hernández, E.N. et al. Differential disruption on glucose and insulin metabolism in two rat models of diet-induced obesity, based on carbohydrates or lipids. Mol Cell Biochem 478, 2481–2488 (2023). https://doi.org/10.1007/s11010-023-04677-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04677-4