Abstract
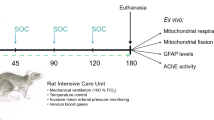
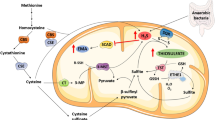
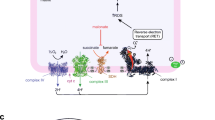
Sodium fluoroacetate (FA) is a metabolic poison that systemically inhibits the tricarboxylic acid (TCA) cycle, causing energy deficiency and ultimately multi-organ failure. It poses a significant threat to society because of its high toxicity, potential use as a chemical weapon and lack of effective antidotal therapy. In this study, we investigated cell-permeable succinate prodrugs as potential treatment for acute FA intoxication. We hypothesized that succinate prodrugs would bypass FA-induced mitochondrial dysfunction, provide metabolic support, and prevent metabolic crisis during acute FA intoxication. To test this hypothesis, rats were exposed to FA (0.75 mg/kg) and treated with the succinate prodrug candidate NV354. Treatment efficacy was evaluated based on cardiac and cerebral mitochondrial respiration, mitochondrial content, metabolic profiles and tissue pathology. In the heart, FA increased concentrations of the TCA metabolite citrate (+ 4.2-fold, p < 0.01) and lowered ATP levels (− 1.9-fold, p < 0.001), confirming the inhibition of the TCA cycle by FA. High-resolution respirometry of cardiac mitochondria further revealed an impairment of mitochondrial complex V (CV)-linked metabolism, as evident by a reduced phosphorylation system control ratio (− 41%, p < 0.05). The inhibition of CV-linked metabolism is a novel mechanism of FA cardiac toxicity, which has implications for drug development and which NV354 was unable to counteract at the given dose. In the brain, FA induced the accumulation of β-hydroxybutyrate (+ 1.4-fold, p < 0.05) and the reduction of mitochondrial complex I (CI)-linked oxidative phosphorylation (OXPHOSCI) (− 20%, p < 0.01), the latter of which was successfully alleviated by NV354. This promising effect of NV354 warrants further investigations to determine its potential neuroprotective effects.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Proudfoot AT, Bradberry SM, Vale JA (2006) Sodium fluoroacetate poisoning. Toxicol Rev 25(4):213–219
Goncharov NV, Jenkins RO, Radilov AS (2006) Toxicology of fluoroacetate: a review, with possible directions for therapy research. J Appl Toxicol: JAT 26(2):148–161
Martínez-Reyes I, Chandel NS (2020) Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 11(1):102
McCranor BJ, Young TD, Tressler J, Jennings L, Irwin J, Alli NA et al (2019) The cardiopulmonary effects of sodium fluoroacetate (1080) in Sprague–Dawley rats. Cogent Biol 5(1):1568669
DeLey Cox VE, Hartog MA, Pueblo E, Racine M, Jennings L, Tressler J et al (2020) Methylene blue and monosodium glutamate improve neurologic signs after fluoroacetate poisoning. Ann N Y Acad Sci 1479(1):196–209
Goncharov N, Glashkina L, Savelieva E, Zinchenko V, Kuznetsov S, Vinokurov M et al (2009) CHAPTER 13 - Fluoroacetate. In: Gupta RC (ed) Handbook of toxicology of chemical warfare agents. Academic Press, San Diego, pp 177–198
Ehinger JK, Piel S, Ford R, Karlsson M, Sjovall F, Frostner EA et al (2016) Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat Commun 7:12317
Jang DH, Piel S, Greenwood JC, Kelly M, Mazandi VM, Ranganathan A et al (2021) Alterations in cerebral and cardiac mitochondrial function in a porcine model of acute carbon monoxide poisoning. Clin Toxicol (Philadelphia, Pa). 59:1–14
Pesta D, Gnaiger E (2012) High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol (Clifton, NJ) 810:25–58
Morota S, Manolopoulos T, Eyjolfsson A, Kimblad P-O, Wierup P, Metzsch C et al (2013) Functional and pharmacological characteristics of permeability transition in isolated human heart mitochondria. PloS One 8(6):e67747
Kirzon MV, Timeĭko VN, Artiushkova VA (1973) Citric acid accumulation in different organs of rabbits and cats with sodium fluoroacetate poisoning. Vopr Med Khimii 19(5):471–475
Bowman RH (1964) Inhibition of citrate metabolism by sodium fluoroacetate in the perfused rat heart and the effect on phosphofructokinase activity and glucose utilization. Biochem J 93(2):13c-c15
Buffa P, Peters RA (1949) The in vivo formation of citrate induced by fluoroacetate and its significance. J Physiol 110(3–4):488–500
Karlsson M, Ehinger JK, Piel S, Sjovall F, Henriksnas J, Hoglund U et al (2016) Changes in energy metabolism due to acute rotenone-induced mitochondrial complex I dysfunction—an in vivo large animal model. Mitochondrion 31:56–62
Kann O, Kovács R (2007) Mitochondria and neuronal activity. Am J Physiol Cell Physiol 292(2):C641–C657
Rowley NM, Madsen KK, Schousboe A, Steve WH (2012) Glutamate and GABA synthesis, release, transport and metabolism as targets for seizure control. Neurochem Int 61(4):546–558
Norwitz NG, Hu MT, Clarke K (2019) the mechanisms by which the Ketone body d-β-hydroxybutyrate may improve the multiple cellular pathologies of Parkinson’s disease. Front Nutr 6:63
Rojas-Morales P, Pedraza-Chaverri J, Tapia E (2020) Ketone bodies, stress response, and redox homeostasis. Redox Biol 29:101395
García-Rodríguez D, Giménez-Cassina A (2021) Ketone bodies in the brain beyond fuel metabolism: from excitability to gene expression and cell signaling. Front Mol Neurosci 14:171
Cartwright MM, Hajja W, Al-Khatib S, Hazeghazam M, Sreedhar D, Li RN et al (2012) Toxigenic and metabolic causes of ketosis and ketoacidotic syndromes. Crit Care Clin 28(4):601–631
Omara F, Sisodia CS (1990) Evaluation of potential antidotes for sodium fluoroacetate in mice. Vet Hum Toxicol 32(5):427–431
Collicchio-Zuanaze RC, Sakate M, Schwartz DS, Trezza E, Crocci AJ (2006) Calcium gluconate and sodium succinate for therapy of sodium fluoroacetate experimental intoxication in cats: clinical and electrocardiographic evaluation. Hum Exp Toxicol 25(4):175–182
Yu Y, Yu J, Yao R, Wang P, Zhang Y, Xiao J et al (2021) Admission serum ionized and total calcium as new predictors of mortality in patients with cardiogenic shock. Biomed Res Int 2021:6612276
Roy A, Taitelman U, Bursztein S (1980) Evaluation of the role of ionized calcium in sodium fluoroacetate (“1080”) poisoning. Toxicol Appl Pharmacol 56(2):216–220
Taitelman U, Roy A, Raikhlin-Eisenkraft B, Hoffer E (1983) The effect of monoacetin and calcium chloride on acid-base balance and survival in experimental sodium fluoroacetate poisoning. Arch Toxicol Suppl 6:222–7
Khellaf A, Garcia NM, Tajsic T, Alam A, Stovell MG, Killen MJ et al (2022) Focally administered succinate improves cerebral metabolism in traumatic brain injury patients with mitochondrial dysfunction. J Cerebral Blood Flow Metabol 42(1):39–55
Stovell MG, Mada MO, Helmy A, Carpenter TA, Thelin EP, Yan JL et al (2018) The effect of succinate on brain NADH/NAD(+) redox state and high energy phosphate metabolism in acute traumatic brain injury. Sci Rep 8(1):11140
Jalloh I, Helmy A, Howe DJ, Shannon RJ, Grice P, Mason A et al (2017) Focally perfused succinate potentiates brain metabolism in head injury patients. J Cerebral Blood Flow Metabol 37(7):2626–2638
Giorgi-Coll S, Amaral AI, Hutchinson PJA, Kotter MR, Carpenter KLH (2017) Succinate supplementation improves metabolic performance of mixed glial cell cultures with mitochondrial dysfunction. Sci Rep 7(1):1003
Oguro H, Iijima K, Takahashi K, Nagai A, Bokura H, Yamaguchi S et al (2004) Successful treatment with succinate in a patient with MELAS. Internal Med (Tokyo, Japan) 43(5):427–431
Paul BD, Snyder SH (2019) Therapeutic applications of cysteamine and cystamine in neurodegenerative and neuropsychiatric diseases. Front Neurol 10:1315
Sun L, Xu S, Zhou M, Wang C, Wu Y, Chan P (2010) Effects of cysteamine on MPTP-induced dopaminergic neurodegeneration in mice. Brain Res 1335:74–82
Gibrat C, Cicchetti F (2011) Potential of cystamine and cysteamine in the treatment of neurodegenerative diseases. Prog Neuropsychopharmacol Biol Psychiatry 35(2):380–389
Mead RJ, Moulden DL, Twigg LE (1985) Significance of sulfhydryl compounds in the manifestation of fluoroacetate toxicity to the rat, brush-tailed possum, woylie and western grey kangaroo. Aust J Biol Sci 38(2):139–149
Hutchens JO, Wagner H et al (1949) The effect of ethanol and various metabolites on fluoroacetate poisoning. J Pharmacol Exp Ther 95(1):62–70
Miller MA (2001) Gender-based differences in the toxicity of pharmaceuticals—the Food and Drug Administration’s perspective. Int J Toxicol 20(3):149–152
Lipnick RL, Cotruvo JA, Hill RN, Bruce RD, Stitzel KA, Walker AP et al (1995) Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem Toxicol 33(3):223–231
Comfort N, Re DB (2017) Sex-specific neurotoxic effects of organophosphate pesticides across the life course. Curr Environ Health Rep 4(4):392–404
Torres-Rojas C, Jones BC (2018) Sex differences in neurotoxicogenetics. Front Genet 9:196
Soldin OP, Mattison DR (2009) Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet 48(3):143–157
Madla CM, Gavins FKH, Merchant HA, Orlu M, Murdan S, Basit AW (2021) Let’s talk about sex: Differences in drug therapy in males and females. Adv Drug Deliv Rev 175:113804
Özdemir BC, Gerard CL, Espinosa da SC (2022) Sex and gender differences in anticancer treatment toxicity: a call for revisiting drug dosing in oncology. Endocrinology 163:6
Romanescu M, Buda V, Lombrea A, Andor M, Ledeti I, Suciu M et al (2022) Sex-related differences in pharmacological response to CNS drugs: a narrative review. J Personal Med 12:6
Kim HI, Lim H, Moon A (2018) Sex differences in cancer: epidemiology. Genet Therapy Biomol Ther (Seoul) 26(4):335–342
Demarest TG, McCarthy MM (2015) Sex differences in mitochondrial (dys)function: implications for neuroprotection. J Bioenerg Biomembr 47(1–2):173–188
Silaidos C, Pilatus U, Grewal R, Matura S, Lienerth B, Pantel J et al (2018) Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol Sex Differ 9(1):34
Demarest TG, Schuh RA, Waddell J, McKenna MC, Fiskum G (2016) Sex-dependent mitochondrial respiratory impairment and oxidative stress in a rat model of neonatal hypoxic-ischemic encephalopathy. J Neurochem 137(5):714–729
Kalimon OJ, Sullivan PG (2021) Sex differences in mitochondrial function following a controlled cortical impact traumatic brain injury in rodents. Front Mol Neurosci 14:753946
Acknowledgements
The authors would like to thank Abliva AB for providing NV354 and Dr. Christopher Petucci and the University of Pennsylvania Metabolomics Core for performing the metabolomics analysis. We would like to thank Drs. David Jett and Shardell Spriggs for their encouragement and guidance and the many members of the CounterAct Program who work tirelessly for our sake.
Funding
This work is supported by the CounterAct Program, National Institute of Neurologic Disorders and Stroke [Kilbaugh; R21 NS103826]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
TJK, SP, JIJ, MK, and JKE contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SP, JIJ, JLW, MH, EE, PKJ, JS, and JNH. The first draft of the manuscript was written by SP, JIJ, and JLW and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
S.P., M.K., J.K.E., E.E. and M.J.H have, or have had, salary from and/or equity interest in Abliva AB. S.P., J.K.E., E.E. and M.J.H have filed patent applications for the use of succinate prodrugs for treatment of lactic acidosis or drug-induced side-effects due to complex I-related impairment of mitochondrial oxidative phosphorylation (WO/2015/155238). S.P., M.K., J.K.E., E.E. and M.J.H additionally have filed patent applications for the use of protected carboxylic acid-based metabolites for treatment of mitochondrial disorders (WO/2017/060400, WO/2017/060418, WO/2017/060422).
Ethics approval
All procedures were approved by the Institutional Animal Care and Use Committee at the Children’s Hospital of Philadelphia and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Piel, S., Janowska, J.I., Ward, J.L. et al. Succinate prodrugs as treatment for acute metabolic crisis during fluoroacetate intoxication in the rat. Mol Cell Biochem 478, 1231–1244 (2023). https://doi.org/10.1007/s11010-022-04589-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04589-9