Abstract
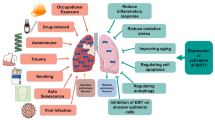
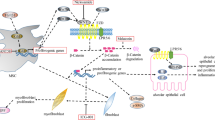
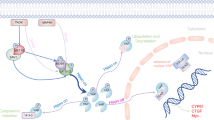
YAP and TAZ are important co-activators of various biological processes in human body. YAP/TAZ plays a vital role in the development of pulmonary fibrosis. Dysregulation of the YAP/TAZ signaling pathway is one of the most important causes of pulmonary fibrosis. Therefore, considering its crucial role, summary of the signal mechanism of YAP/TAZ is of certain guiding significance for the research of YAP/TAZ as a therapeutic target. The present review provided a detailed introduction to various YAP/TAZ-related signaling pathways and clarified the specific role of YAP/TAZ in these pathways. In the meantime, we summarized and evaluated possible applications of YAP/TAZ in the treatment of pulmonary fibrosis. Overall, our study is of guiding significance for future research on the functional mechanism of YAP/TAZ underlying lung diseases as well as for identification of novel therapeutic targets specific to pulmonary fibrosis.





Similar content being viewed by others
Data availability
The data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding author on reasonable request.
References
Chua F, Sly PD, Laurent GJ (2005) Pediatric lung disease: from proteinases to pulmonary fibrosis. Pediatr Pulmonol 39:392–401. https://doi.org/10.1002/ppul.20171
Borensztajn K, Crestani B, Kolb M (2013) Idiopathic pulmonary fibrosis: from epithelial injury to biomarkers–insights from the bench side. Respiration 86:441–452. https://doi.org/10.1159/000357598
Marshall DC, Salciccioli JD, Shea BS, Akuthota P (2018) Trends in mortality from idiopathic pulmonary fibrosis in the European Union: an observational study of the WHO mortality database from 2001–2013. Eur Respir J. https://doi.org/10.1183/13993003.01603-2017
Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D, Pneumonias AECoII (2013) An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188:733–748. https://doi.org/10.1164/rccm.201308-1483ST
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ, Fibrosis AEJACoIP (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183:788–824. https://doi.org/10.1164/rccm.2009-040GL
Mason DP, Brizzio ME, Alster JM, McNeill AM, Murthy SC, Budev MM, Mehta AC, Minai OA, Pettersson GB, Blackstone EH (2007) Lung transplantation for idiopathic pulmonary fibrosis. Ann Thorac Surg 84:1121–1128. https://doi.org/10.1016/j.athoracsur.2007.04.096
Vancheri C, Failla M, Crimi N, Raghu G (2010) Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J 35:496–504. https://doi.org/10.1183/09031936.00077309
Kim HJ, Perlman D, Tomic R (2015) Natural history of idiopathic pulmonary fibrosis. Respir Med 109:661–670. https://doi.org/10.1016/j.rmed.2015.02.002
Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR (2014) Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2:566–572. https://doi.org/10.1016/S2213-2600(14)70101-8
Hutchinson J, Fogarty A, Hubbard R, McKeever T (2015) Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J 46:795–806. https://doi.org/10.1183/09031936.00185114
Pereira CAC, Baddini-Martinez JA, Baldi BG, Jezler SFO, Rubin AS, Alves RLR, Zonzin GA, Quaresma M, Trampisch M, Rabahi MF (2019) Safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis in Brazil. J Bras Pneumol 45:e20180414. https://doi.org/10.1590/1806-3713/e20180414
Gao F, Wu J, Niu S, Sun T, Li F, Bai Y, Jin L, Lin L, Shi Q, Zhu LM, Du L (2019) Biodegradable, pH-sensitive hollow mesoporous organosilica nanoparticle (HMON) with controlled release of pirfenidone and ultrasound-target-microbubble-destruction (UTMD) for pancreatic cancer treatment. Theranostics 9:6002–6018. https://doi.org/10.7150/thno.36135
Wilson KC, Raghu G (2015) The 2015 guidelines for idiopathic pulmonary fibrosis: an important chapter in the evolution of the management of patients with IPF. Eur Respir J 46:883–886. https://doi.org/10.1183/13993003.01335-2015
Oldham JM, Ma SF, Martinez FJ, Anstrom KJ, Raghu G, Schwartz DA, Valenzi E, Witt L, Lee C, Vij R, Huang Y, Strek ME, Noth I, Investigators IP (2015) TOLLIP, MUC5B, and the response to N-acetylcysteine among individuals with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 192:1475–1482. https://doi.org/10.1164/rccm.201505-1010OC
Egan JJ (2011) Follow-up and nonpharmacological management of the idiopathic pulmonary fibrosis patient. Eur Respir Rev 20:114–117. https://doi.org/10.1183/09059180.00001811
Necki M, Pandel A, Urlik M, Antonczyk R, Latos M, Gaweda M, Stacel T, Wajda-Pokrontka M, Zawadzki F, Okienica M, Przybylowski P, Zembala M, Ochman M (2020) The impact of airway complications on survival among lung transplant recipients. Transplant Proc. https://doi.org/10.1016/j.transproceed.2020.03.051
Han L, Liu D, Li Z, Tian N, Han Z, Wang G, Fu Y, Guo Z, Zhu Z, Du C, Tian Y (2015) HOXB1 is a tumor suppressor gene regulated by miR-3175 in glioma. PLoS ONE 10:e0142387. https://doi.org/10.1371/journal.pone.0142387
Campbell KN, Wong JS, Gupta R, Asanuma K, Sudol M, He JC, Mundel P (2013) Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J Biol Chem 288:17057–17062. https://doi.org/10.1074/jbc.C113.457390
Komuro A, Nagai M, Navin NE, Sudol M (2003) WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem 278:33334–33341. https://doi.org/10.1074/jbc.M305597200
Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA (2006) Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA 103:12405–12410. https://doi.org/10.1073/pnas.0605579103
McDonald CB, McIntosh SK, Mikles DC, Bhat V, Deegan BJ, Seldeen KL, Saeed AM, Buffa L, Sudol M, Nawaz Z, Farooq A (2011) Biophysical analysis of binding of WW domains of the YAP2 transcriptional regulator to PPXY motifs within WBP1 and WBP2 adaptors. Biochemistry 50:9616–9627. https://doi.org/10.1021/bi201286p
Xie H, Wu L, Deng Z, Huo Y, Cheng Y (2018) Emerging roles of YAP/TAZ in lung physiology and diseases. Life Sci 214:176–183. https://doi.org/10.1016/j.lfs.2018.10.062
Pan H, Liao M, Song P (2018) YAP/TAZ regulates multiple signal pathways in the genesis and development of hepatic fibrosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 43:313–319. https://doi.org/10.11817/j.issn.1672-7347.2018.03.013
Byrne AJ, Maher TM, Lloyd CM (2016) Pulmonary macrophages: a new therapeutic pathway in fibrosing lung disease? Trends Mol Med 22:303–316. https://doi.org/10.1016/j.molmed.2016.02.004
Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ (2015) Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308:L344–L357. https://doi.org/10.1152/ajplung.00300.2014
Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, Stripp BR, Whitsett JA (2016) Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1:e90558. https://doi.org/10.1172/jci.insight.90558
Noguchi S, Saito A, Mikami Y, Urushiyama H, Horie M, Matsuzaki H, Takeshima H, Makita K, Miyashita N, Mitani A, Jo T, Yamauchi Y, Terasaki Y, Nagase T (2017) TAZ contributes to pulmonary fibrosis by activating profibrotic functions of lung fibroblasts. Sci Rep 7:42595. https://doi.org/10.1038/srep42595
Gokey JJ, Sridharan A, Xu Y, Green J, Carraro G, Stripp BR, Perl AT, Whitsett JA (2018) Active epithelial Hippo signaling in idiopathic pulmonary fibrosis. JCI Insight. https://doi.org/10.1172/jci.insight.98738
Mitani A, Nagase T, Fukuchi K, Aburatani H, Makita R, Kurihara H (2009) Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am J Respir Crit Care Med 180:326–338. https://doi.org/10.1164/rccm.200812-1827OC
Jorgenson AJ, Choi KM, Sicard D, Smith KM, Hiemer SE, Varelas X, Tschumperlin DJ (2017) TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am J Physiol Cell Physiol 312:C277–C285. https://doi.org/10.1152/ajpcell.00205.2016
Mo JS, Park HW, Guan KL (2014) The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 15:642–656. https://doi.org/10.15252/embr.201438638
Zhao B, Li L, Lei Q, Guan KL (2010) The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 24:862–874. https://doi.org/10.1101/gad.1909210
Zhao B, Tumaneng K, Guan KL (2011) The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 13:877–883. https://doi.org/10.1038/ncb2303
Yu FX, Guan KL (2013) The Hippo pathway: regulators and regulations. Genes Dev 27:355–371. https://doi.org/10.1101/gad.210773.112
Hansen CG, Moroishi T, Guan KL (2015) YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol 25:499–513. https://doi.org/10.1016/j.tcb.2015.05.002
Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH (2005) The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24:2076–2086. https://doi.org/10.1038/sj.onc.1208445
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21:2747–2761. https://doi.org/10.1101/gad.1602907
Praskova M, Xia F, Avruch J (2008) MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol 18:311–321. https://doi.org/10.1016/j.cub.2008.02.006
Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D (2007) Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130:1120–1133. https://doi.org/10.1016/j.cell.2007.07.019
Oh H, Irvine KD (2008) In vivo regulation of Yorkie phosphorylation and localization. Development 135:1081–1088. https://doi.org/10.1242/dev.015255
Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei QY, Guan KL (2010) The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem 285:37159–37169. https://doi.org/10.1074/jbc.M110.152942
Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, Yaffe MB (2000) TAZ: a novel transcriptional co-activator regulated by interactions with 14–3-3 and PDZ domain proteins. EMBO J 19:6778–6791. https://doi.org/10.1093/emboj/19.24.6778
Maejima Y, Kyoi S, Zhai P, Liu T, Li H, Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, Lim DS, Isobe M, Sadoshima J (2013) Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med 19:1478–1488. https://doi.org/10.1038/nm.3322
Hudgens-Haney ME, Ethridge LE, McDowell JE, Keedy SK, Pearlson GD, Tamminga CA, Keshavan MS, Sweeney JA, Clementz BA (2018) Psychosis subgroups differ in intrinsic neural activity but not task-specific processing. Schizophr Res 195:222–230. https://doi.org/10.1016/j.schres.2017.08.023
Thompson BJ, Sahai E (2015) MST kinases in development and disease. J Cell Biol 210:871–882. https://doi.org/10.1083/jcb.201507005
Oudhoff MJ, Freeman SA, Couzens AL, Antignano F, Kuznetsova E, Min PH, Northrop JP, Lehnertz B, Barsyte-Lovejoy D, Vedadi M, Arrowsmith CH, Nishina H, Gold MR, Rossi FM, Gingras AC, Zaph C (2013) Control of the hippo pathway by Set7-dependent methylation of Yap. Dev Cell 26:188–194. https://doi.org/10.1016/j.devcel.2013.05.025
Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan F, Meng S, Wang Y, Yuan Z, Bi W (2014) SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene 33:1468–1474. https://doi.org/10.1038/onc.2013.88
Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, Shao DD, Schumacher SE, Weir BA, Vazquez F, Cowley GS, Root DE, Mesirov JP, Beroukhim R, Kuo CJ, Goessling W, Hahn WC (2012) beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151:1457–1473. https://doi.org/10.1016/j.cell.2012.11.026
Kohli P, Bartram MP, Habbig S, Pahmeyer C, Lamkemeyer T, Benzing T, Schermer B, Rinschen MM (2014) Label-free quantitative proteomic analysis of the YAP/TAZ interactome. Am J Physiol Cell Physiol 306:C805–C818. https://doi.org/10.1152/ajpcell.00339.2013
Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS (2006) Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366:2–16. https://doi.org/10.1016/j.gene.2005.10.018
Sibilia M, Kroismayr R, Lichtenberger BM, Natarajan A, Hecking M, Holcmann M (2007) The epidermal growth factor receptor: from development to tumorigenesis. Differentiation 75:770–787. https://doi.org/10.1111/j.1432-0436.2007.00238.x
Venkataraman T, Frieman MB (2017) The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antiviral Res 143:142–150. https://doi.org/10.1016/j.antiviral.2017.03.022
Schlessinger J (2004) Common and distinct elements in cellular signaling via EGF and FGF receptors. Science 306:1506–1507. https://doi.org/10.1126/science.1105396
Reddy BV, Irvine KD (2013) Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell 24:459–471. https://doi.org/10.1016/j.devcel.2013.01.020
Yang CH, Chou HC, Fu YN, Yeh CL, Cheng HW, Chang IC, Liu KJ, Chang GC, Tsai TF, Tsai SF, Liu HP, Wu YC, Chen YT, Huang SF, Chen YR (2015) EGFR over-expression in non-small cell lung cancers harboring EGFR mutations is associated with marked down-regulation of CD82. Biochim Biophys Acta 1852:1540–1549. https://doi.org/10.1016/j.bbadis.2015.04.020
Rothschild SI, Gautschi O, Haura EB, Johnson FM (2010) Src inhibitors in lung cancer: current status and future directions. Clin Lung Cancer 11:238–242. https://doi.org/10.3816/CLC.2010.n.030
Antoniou KM, Margaritopoulos GA, Soufla G, Symvoulakis E, Vassalou E, Lymbouridou R, Samara KD, Kappou D, Spandidos DA, Siafakas NM (2010) Expression analysis of Akt and MAPK signaling pathways in lung tissue of patients with idiopathic pulmonary fibrosis (IPF). J Recept Signal Transduct Res 30:262–269. https://doi.org/10.3109/10799893.2010.489227
Nho RS, Polunovsky V (2013) Translational control of the fibroblast-extracellular matrix association: an application to pulmonary fibrosis. Translation (Austin) 1:e23934. https://doi.org/10.4161/trla.23934
You B, Yang YL, Xu Z, Dai Y, Liu S, Mao JH, Tetsu O, Li H, Jablons DM, You L (2015) Inhibition of ERK1/2 down-regulates the Hippo/YAP signaling pathway in human NSCLC cells. Oncotarget 6:4357–4368. https://doi.org/10.18632/oncotarget.2974
McGowan M, Kleinberg L, Halvorsen AR, Helland A, Brustugun OT (2017) NSCLC depend upon YAP expression and nuclear localization after acquiring resistance to EGFR inhibitors. Genes Cancer 8:497–504. https://doi.org/10.18632/genesandcancer.136
Polakis P (2007) The many ways of Wnt in cancer. Curr Opin Genet Dev 17:45–51. https://doi.org/10.1016/j.gde.2006.12.007
Wang Y, Li YP, Paulson C, Shao JZ, Zhang X, Wu M, Chen W (2014) Wnt and the Wnt signaling pathway in bone development and disease. Front Biosci (Landmark Ed) 19:379–407. https://doi.org/10.2741/4214
Klaus A, Birchmeier W (2008) Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8:387–398. https://doi.org/10.1038/nrc2389
Myung SJ, Yoon JH, Gwak GY, Kim W, Lee JH, Kim KM, Shin CS, Jang JJ, Lee SH, Lee SM, Lee HS (2007) Wnt signaling enhances the activation and survival of human hepatic stellate cells. FEBS Lett 581:2954–2958. https://doi.org/10.1016/j.febslet.2007.05.050
Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL (2015) Alternative Wnt signaling activates YAP/TAZ. Cell 162:780–794. https://doi.org/10.1016/j.cell.2015.07.013
Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S (2014) YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158:157–170. https://doi.org/10.1016/j.cell.2014.06.013
van Amerongen R (2012) Alternative Wnt pathways and receptors. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a007914
Cheng JH, She H, Han YP, Wang J, Xiong S, Asahina K, Tsukamoto H (2008) Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 294:G39–49. https://doi.org/10.1152/ajpgi.00263.2007
Ferrari N, Ranftl R, Chicherova I, Slaven ND, Moeendarbary E, Farrugia AJ, Lam M, Semiannikova M, Westergaard MCW, Tchou J, Magnani L, Calvo F (2019) Dickkopf-3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer-associated fibroblasts. Nat Commun 10:130. https://doi.org/10.1038/s41467-018-07987-0
Sun Z, Gong X, Zhu H, Wang C, Xu X, Cui D, Qian W, Han X (2014) Inhibition of Wnt/beta-catenin signaling promotes engraftment of mesenchymal stem cells to repair lung injury. J Cell Physiol 229:213–224. https://doi.org/10.1002/jcp.24436
Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S, Wu GS, Wu K (2015) Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett 369:20–27. https://doi.org/10.1016/j.canlet.2015.07.048
Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, Lowe JB, Phan SH (2009) Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol 174:1745–1755. https://doi.org/10.2353/ajpath.2009.080618
Zhang X, Xu Y, Chen JM, Liu C, Du GL, Zhang H, Chen GF, Jiang SL, Liu CH, Mu YP, Liu P (2017) Huang Qi Decoction prevents BDL-induced liver fibrosis through inhibition of Notch signaling activation. Am J Chin Med 45:85–104. https://doi.org/10.1142/S0192415X17500070
Edeling M, Ragi G, Huang S, Pavenstadt H, Susztak K (2016) Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol 12:426–439. https://doi.org/10.1038/nrneph.2016.54
Zhou X, Chen X, Cai JJ, Chen LZ, Gong YS, Wang LX, Gao Z, Zhang HQ, Huang WJ, Zhou H (2015) Relaxin inhibits cardiac fibrosis and endothelial-mesenchymal transition via the Notch pathway. Drug Des Devel Ther 9:4599–4611. https://doi.org/10.2147/DDDT.S85399
Dees C, Tomcik M, Zerr P, Akhmetshina A, Horn A, Palumbo K, Beyer C, Zwerina J, Distler O, Schett G, Distler JH (2011) Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis 70:1304–1310. https://doi.org/10.1136/ard.2010.134742
Rayon T, Menchero S, Nieto A, Xenopoulos P, Crespo M, Cockburn K, Canon S, Sasaki H, Hadjantonakis AK, de la Pompa JL, Rossant J, Manzanares M (2014) Notch and hippo converge on Cdx2 to specify the trophectoderm lineage in the mouse blastocyst. Dev Cell 30:410–422. https://doi.org/10.1016/j.devcel.2014.06.019
Ferretti A, Monaco E, Vadala A (2014) Rotatory instability of the knee after ACL tear and reconstruction. J Orthop Traumatol 15:75–79. https://doi.org/10.1007/s10195-013-0254-y
Leung CY, Zernicka-Goetz M (2013) Angiomotin prevents pluripotent lineage differentiation in mouse embryos via Hippo pathway-dependent and -independent mechanisms. Nat Commun 4:2251. https://doi.org/10.1038/ncomms3251
Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, Makita R, Kurihara H, Morin-Kensicki EM, Nojima H, Rossant J, Nakao K, Niwa H, Sasaki H (2009) The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 16:398–410. https://doi.org/10.1016/j.devcel.2009.02.003
Home P, Saha B, Ray S, Dutta D, Gunewardena S, Yoo B, Pal A, Vivian JL, Larson M, Petroff M, Gallagher PG, Schulz VP, White KL, Golos TG, Behr B, Paul S (2012) Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc Natl Acad Sci USA 109:7362–7367. https://doi.org/10.1073/pnas.1201595109
Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A (2007) Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development 134:3827–3836. https://doi.org/10.1242/dev.010223
Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J (2005) Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132:2093–2102. https://doi.org/10.1242/dev.01801
Li Y, Hibbs MA, Gard AL, Shylo NA, Yun K (2012) Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells 30:741–752. https://doi.org/10.1002/stem.1030
Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, Sticht C, Tomasi ML, Delogu S, Evert M, Fan B, Ribback S, Jiang L, Brozzetti S, Bergmann F, Dombrowski F, Schirmacher P, Calvisi DF, Breuhahn K (2013) Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology 144(1530–1542):e12. https://doi.org/10.1053/j.gastro.2013.02.009
Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD (2014) Hippo pathway activity influences liver cell fate. Cell 157:1324–1338. https://doi.org/10.1016/j.cell.2014.03.060
Tchorz JS, Kinter J, Muller M, Tornillo L, Heim MH, Bettler B (2009) Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology 50:871–879. https://doi.org/10.1002/hep.23048
Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ (2012) Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18:572–579. https://doi.org/10.1038/nm.2667
Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR (2007) YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17:2054–2060. https://doi.org/10.1016/j.cub.2007.10.039
Totaro A, Castellan M, Di Biagio D, Piccolo S (2018) Crosstalk between YAP/TAZ and Notch signaling. Trends Cell Biol 28:560–573. https://doi.org/10.1016/j.tcb.2018.03.001
Song GY, Huang B, Dong HY, Cheng Q, Cui TJ (2016) Broadband focusing acoustic lens based on fractal metamaterials. Sci Rep 6:35929. https://doi.org/10.1038/srep35929
Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I, Rifkin DB (1997) TGF-beta latency: biological significance and mechanisms of activation. Stem Cells 15:190–197. https://doi.org/10.1002/stem.150190
Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB (1997) Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int 51:1376–1382. https://doi.org/10.1038/ki.1997.188
Wu M, Chen G, Li YP (2016) TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4:16009. https://doi.org/10.1038/boneres.2016.9
Martelossi Cebinelli GC, Paiva Trugilo K, Badaro Garcia S, Brajao de Oliveira K (2016) TGF-beta1 functional polymorphisms: a review. Eur Cytokine Netw 27:81–89. https://doi.org/10.1684/ecn.2016.0382
Qin Z, Xia W, Fisher GJ, Voorhees JJ, Quan T (2018) YAP/TAZ regulates TGF-beta/Smad3 signaling by induction of Smad7 via AP-1 in human skin dermal fibroblasts. Cell Commun Signal 16:18. https://doi.org/10.1186/s12964-018-0232-3
Beyer TA, Weiss A, Khomchuk Y, Huang K, Ogunjimi AA, Varelas X, Wrana JL (2013) Switch enhancers interpret TGF-beta and Hippo signaling to control cell fate in human embryonic stem cells. Cell Rep 5:1611–1624. https://doi.org/10.1016/j.celrep.2013.11.021
Narimatsu M, Samavarchi-Tehrani P, Varelas X, Wrana JL (2015) Distinct polarity cues direct Taz/Yap and TGFbeta receptor localization to differentially control TGFbeta-induced Smad signaling. Dev Cell 32:652–656. https://doi.org/10.1016/j.devcel.2015.02.019
Grannas K, Arngarden L, Lonn P, Mazurkiewicz M, Blokzijl A, Zieba A, Soderberg O (2015) Crosstalk between Hippo and TGFbeta: subcellular localization of YAP/TAZ/Smad complexes. J Mol Biol 427:3407–3415. https://doi.org/10.1016/j.jmb.2015.04.015
Zhang J, Smolen GA, Haber DA (2008) Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res 68:2789–2794. https://doi.org/10.1158/0008-5472.CAN-07-6205
Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N (2009) Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16:425–438. https://doi.org/10.1016/j.ccr.2009.09.026
Zeng F, Miyazawa T, Kloepfer LA, Harris RC (2018) ErbB4 deletion accelerates renal fibrosis following renal injury. Am J Physiol Renal Physiol 314:F773–F787. https://doi.org/10.1152/ajprenal.00260.2017
Nakatani K, Maehama T, Nishio M, Goto H, Kato W, Omori H, Miyachi Y, Togashi H, Shimono Y, Suzuki A (2017) Targeting the Hippo signalling pathway for cancer treatment. J Biochem 161:237–244. https://doi.org/10.1093/jb/mvw074
Gibault F, Bailly F, Corvaisier M, Coevoet M, Huet G, Melnyk P, Cotelle P (2017) Molecular features of the YAP inhibitor verteporfin: synthesis of hexasubstituted dipyrrins as potential inhibitors of YAP/TAZ, the downstream effectors of the Hippo pathway. ChemMedChem 12:954–961. https://doi.org/10.1002/cmdc.201700063
Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D (2012) Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev 26:1300–1305. https://doi.org/10.1101/gad.192856.112
Zhao J, Shi W, Chen H, Warburton D (2000) Smad7 and Smad6 differentially modulate transforming growth factor beta -induced inhibition of embryonic lung morphogenesis. J Biol Chem 275:23992–23997. https://doi.org/10.1074/jbc.M002433200
Piguet PF, Vesin C (1994) Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J 7:515–518. https://doi.org/10.1183/09031936.94.07030515
Tan GH, Dutton CM, Bahn RS (1996) Interleukin-1 (IL-1) receptor antagonist and soluble IL-1 receptor inhibit IL-1-induced glycosaminoglycan production in cultured human orbital fibroblasts from patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab 81:449–452. https://doi.org/10.1210/jcem.81.2.8636247
Sueoka N, Sueoka E, Miyazaki Y, Okabe S, Kurosumi M, Takayama S, Fujiki H (1998) Molecular pathogenesis of interstitial pneumonitis with TNF-alpha transgenic mice. Cytokine 10:124–131. https://doi.org/10.1006/cyto.1997.0267
Venkatesan N, Pini L, Ludwig MS (2004) Changes in Smad expression and subcellular localization in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 287:L1342–L1347. https://doi.org/10.1152/ajplung.00035.2004
Higashiyama H, Yoshimoto D, Okamoto Y, Kikkawa H, Asano S, Kinoshita M (2007) Receptor-activated Smad localisation in bleomycin-induced pulmonary fibrosis. J Clin Pathol 60:283–289. https://doi.org/10.1136/jcp.2006.037606
Newton CA, Zhang D, Oldham JM, Kozlitina J, Ma SF, Martinez FJ, Raghu G, Noth I, Garcia CK (2019) Telomere length and use of immunosuppressive medications in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 200:336–347. https://doi.org/10.1164/rccm.201809-1646OC
Hozumi H, Hasegawa H, Miyashita K, Yasui H, Suzuki Y, Kono M, Karayama M, Furuhashi K, Hashimoto D, Enomoto N, Fujisawa T, Inui N, Nakamura Y, Yokomura K, Nakamura H, Suda T (2019) Efficacy of corticosteroid and intravenous cyclophosphamide in acute exacerbation of idiopathic pulmonary fibrosis: a propensity score-matched analysis. Respirology 24:792–798. https://doi.org/10.1111/resp.13506
Biondi NL, Samiratedu MM, Highsmith E, Rosenblum A, McGrady K, Knepper S, Bowers R (2019) the impact of interprofessional monitoring and education on the usage of systemic glucocorticoids in acute exacerbations of chronic obstructive pulmonary disease: a retrospective. Medicat Use Rev Cureus 11:e6224. https://doi.org/10.7759/cureus.6224
Obeidat M, Faiz A, Li X, van den Berge M, Hansel NN, Joubert P, Hao K, Brandsma CA, Rafaels N, Mathias R, Ruczinski I, Beaty TH, Barnes KC, Man SFP, Pare PD, Sin DD (2019) The pharmacogenomics of inhaled corticosteroids and lung function decline in COPD. Eur Respir J. https://doi.org/10.1183/13993003.00521-2019
Kulshrestha R, Pandey A, Jaggi A, Bansal S (2020) Beneficial effects of N-acetylcysteine on protease-antiprotease balance in attenuating bleomycin-induced pulmonary fibrosis in rats. Iran J Basic Med Sci 23:396–405. https://doi.org/10.22038/IJBMS.2020.39031.9261
Salisbury ML, Conoscenti CS, Culver DA, Yow E, Neely ML, Bender S, Hartmann N, Palmer SM, Leonard TB, Investigators I-PR (2020) Antifibrotic drug use in patients with IPF: data from the IPF-PRO registry. Ann Am Thorac Soc. https://doi.org/10.1513/AnnalsATS.201912-880OC
Gercel G, Aksu B, Ozkanli S, Uzun H, Aksu F, Ozatman E, Durakbasa CU (2020) Investigation of Bosentan’s effects on pulmonary contusion created by blunt thoracic trauma in rats. Eur J Pediatr Surg 30:71–78. https://doi.org/10.1055/s-0039-1697908
Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, Vinarsky V, Gonzalez-Celeiro M, Nunna N, Hariri LP, Camargo F, Ellisen LW, Rajagopal J (2014) Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell 30:151–165. https://doi.org/10.1016/j.devcel.2014.06.004
Liu Z, Wu H, Jiang K, Wang Y, Zhang W, Chu Q, Li J, Huang H, Cai T, Ji H, Yang C, Tang N (2016) MAPK-Mediated YAP activation controls mechanical-tension-induced pulmonary alveolar regeneration. Cell Rep 16:1810–1819. https://doi.org/10.1016/j.celrep.2016.07.020
Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, Tzahor E (2017) The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547:179–184. https://doi.org/10.1038/nature22978
Ni X, Tao J, Barbi J, Chen Q, Park BV, Li Z, Zhang N, Lebid A, Ramaswamy A, Wei P, Zheng Y, Zhang X, Wu X, Vignali P, Yang CP, Li H, Pardoll D, Lu L, Pan D, Pan F (2018) YAP is essential for Treg-mediated suppression of antitumor immunity. Cancer Discov 8:1026–1043. https://doi.org/10.1158/2159-8290.CD-17-1124
Liu B, Zheng Y, Yin F, Yu J, Silverman N, Pan D (2016) Toll receptor-mediated Hippo signaling controls innate immunity in Drosophila. Cell 164:406–419. https://doi.org/10.1016/j.cell.2015.12.029
Boro M, Singh V, Balaji KN (2016) Mycobacterium tuberculosis-triggered Hippo pathway orchestrates CXCL1/2 expression to modulate host immune responses. Sci Rep 6:37695. https://doi.org/10.1038/srep37695
Wang S, Xie F, Chu F, Zhang Z, Yang B, Dai T, Gao L, Wang L, Ling L, Jia J, van Dam H, Jin J, Zhang L, Zhou F (2017) YAP antagonizes innate antiviral immunity and is targeted for lysosomal degradation through IKKvarepsilon-mediated phosphorylation. Nat Immunol 18:733–743. https://doi.org/10.1038/ni.3744
Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, Xiong X, Hong L, Xie C, Gao J, Shi Y, Peng J, Johnson RL, Xiao N, Lu L, Han J, Zhou D, Chen L (2018) Publisher Correction: the transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol 19:1036. https://doi.org/10.1038/s41590-018-0055-9
Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, Xiong X, Hong L, Xie C, Gao J, Shi Y, Peng J, Johnson RL, Xiao N, Lu L, Han J, Zhou D, Chen L (2017) The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol 18:800–812. https://doi.org/10.1038/ni.3748
Acknowledgements
Not applicable.
Funding
This study was supported by the funds from Zhejiang Provincial Science and Technology Projects (Grant No. 2018C3710) and Medical Science and Technology Project of Zhejiang Province (Grant No. 2020KY321).
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they no conflicts of interest.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, T., Ma, Z., Wang, H. et al. YAP/TAZ affects the development of pulmonary fibrosis by regulating multiple signaling pathways. Mol Cell Biochem 475, 137–149 (2020). https://doi.org/10.1007/s11010-020-03866-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03866-9