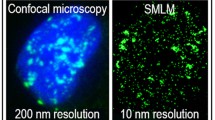
Abstract
In response to DNA double-strand breaks (DSB), histone H2AX is phosphorylated around the lesion by a feed forward signal amplification loop, originating γH2AX foci detectable by immunofluorescence and confocal microscopy as elliptical areas of uniform intensity. We exploited the significant increase in resolution (~ × 10) provided by single-molecule localization microscopy (SMLM) to investigate at nanometer scale the distribution of γH2AX signals either endogenous (controls) or induced by the radiomimetic bleomycin (BLEO) in HeLa cells. In both conditions, clustered substructures (nanofoci) confined to γH2AX foci and scattered nanofoci throughout the remnant nuclear area were detected. SR-Tesseler software (Voronoï tessellation-based segmentation) was combined with a custom Python script to first separate clustered nanofoci inside γH2AX foci from scattered nanofoci, and then to perform a cluster analysis upon each nanofoci type. Compared to controls, γH2AX foci in BLEO-treated nuclei presented on average larger areas (0.41 versus 0.19 µm2), more nanofoci per focus (22.7 versus 13.2) and comparable nanofoci densities (~ 60 nanofoci/µm2). Scattered γH2AX nanofoci were equally present (~ 3 nanofoci/µm2), suggesting an endogenous origin. BLEO-treated cells were challenged with specific inhibitors of canonical H2AX kinases, namely: KU-55933, VE-821 and NU-7026 for ATM, ATR and DNA-PK, respectively. Under treatment with pooled inhibitors, clustered nanofoci vanished from super-resolution images while scattered nanofoci decreased (~ 50%) in density. Residual scattered nanofoci could reflect, among other alternatives, H2AX phosphorylation mediated by VRK1, a recently described non-canonical H2AX kinase. In addition to H2AX findings, an analytical approach to quantify clusters of highly differing density from SMLM data is put forward.




Similar content being viewed by others
Abbreviations
- γH2AX:
-
Histone H2AX phosphorylated on serine 139
- ATM:
-
Ataxia telangiectasia mutated
- ATR:
-
ATM and Rad3-related
- BLEO:
-
Bleomycin
- CLSM:
-
Confocal laser scanning microscopy
- DDR:
-
DNA damage response
- DNA-PK:
-
DNA-dependent protein kinase
- DSB:
-
Double-strand break
- dSTORM:
-
Direct stochastic optical reconstruction microscopy
- IR:
-
Ionizing radiation
- LET:
-
Linear energy transfer
- MAPK:
-
Mitogen-activated protein kinase
- PI3K:
-
Phosphatidylinositol 3-kinase
- ROI:
-
Region of interest
- SIM:
-
Structured illumination microscopy
- SMLM:
-
Single-molecule localization microscopy
- STED:
-
Stimulated emission depletion microscopy
- TSS:
-
Transcription start site
References
Rogakou E, Pilch D, Orr A, Ivanova V, Bonner W (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273:5858–5868
Ward I, Chen J (2001) Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem 276:47759–44762
Kinner A, Wu W, Staudt C, Lliakis G (2008) γH2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res 36:5678–5694
Rogakou E, Boon C, Redon C, Bonner W (1999) Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 146:905–915
Celeste A, Difilippantonio S, Difilippantonio M, Fernandez-Capetillo O, Pilch D, Sedelnikova O, Eckhaus M, Ried T, Bonner W, Nussenzweig A (2003) Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 5:675–679
Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461:1071–1078
Gelot C, Magdalou I, Lopez BS (2015) Replication stress in mammalian cells and its consequences for mitosis. Genes 6:267–298
Vitelli V, Galbiati A, Iannelli F, Pessina F, Sharma S, d’Adda di Fagagna F (2017) Recent advancements in DNA damage-transcription crosstalk and high-resolution mapping of DNA Breaks. Annu Rev Genomics Hum Genet 18:87–113
Bouwman B, Crosetto N (2018) Endogenous DNA double-strand breaks during DNA transactions: emerging insights and methods for genome-wide profiling. Genes (Basel) 9:E632
Chen HT, Bhandoola A, Difilippantonio MJ, Zhu J, Brown MJ, Tai X, Rogakou EP, Brotz TM, Bonner WM, Ried T, Nussenzweig A (2000) Response to RAG-mediated V(D)J cleavage by NBS1 and γ-H2AX. Science 290:1962–1965
Umezawa H, Maeda K, Takeuchi T, Okami Y (1966) New antibiotics, bleomycin A and B. J Antibiot (Tokyo) 19:200–209
Chen J, Stubbe J (2005) Bleomycins: toward better therapeutics. Nat Rev Cancer 5:102–112
Henner WD, Grunberg SM, Haseltine WA (1982) Sites and structure of gamma radiation-induced DNA strand breaks. J Biol Chem 257:11750–11754
Oike T, Niimi A, Okonogi N, Murata K, Matsumura A, Noda SE, Kobayashi D, Iwanaga M, Tsuchida K, Kanai T, Ohno T, Shibata A, Nakano T (2016) Visualization of complex DNA double-strand breaks in a tumor treated with carbon ion radiotherapy. Sci Rep 6:22275
Regulus P, Duroux B, Bayle PA, Favier A, Cadet J, Ravanat JL (2007) Oxidation of the sugar moiety of DNA by ionizing radiation or bleomycin could induce the formation of a cluster DNA lesion. Proc Natl Acad Sci USA 104:14032–14037
Mavragani IV, Nikitaki Z, Souli MP, Aziz A, Nowsheen S, Aziz K, Rogakou E, Georgakilas AG (2017) Complex DNA damage: a route to radiation-induced genomic instability and carcinogenesis. Cancers (Basel) 9:91. https://doi.org/10.3390/cancers9070091
Schipler A, Iliakis G (2013) DNA double-strand-break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice. Nucleic Acids Res 41:7589–7605
Nickoloff JA, Sharma N, Taylor L (2020) Clustered DNA double-strand breaks: biological effects and relevance to cancer radiotherapy. Genes (Basel) 11(1):99
Liddle P, Lafon-Hughes L, Di Tomaso MV, Reyes-Ábalos AL, Jara J, Cerda M, Härtel S, Folle GA (2014) Bleomycin-induced γH2AX foci map preferentially to replicating domains in CHO9 interphase nuclei. Chromosome Res 22:463–481
Schermelleh L, Heintzmann R, Leonhardt H (2010) A guide to super-resolution fluorescence microscopy. J Cell Biol 190:165–175
Reindl J, Drexler GA, Girst S, Greubel C, Siebenwirth C, Drexler SE, Dollinger G, Friedl AA (2015) Nanoscopic exclusion between Rad51 and 53BP1 after ion irradiation in human HeLa cells. Phys Biol 12:066005
Reindl J, Girst S, Walsh DW, Greubel C, Schwarz B, Siebenwirth C, Drexler GA, Friedl AA, Dollinger G (2017) Chromatin organization revealed by nanostructure of irradiation induced γH2AX, 53BP1 and Rad51 foci. Sci Rep 7:40616
Natale F, Rapp A, Yu W, Maiser A, Harz H, Scholl A, Grulich S, Anton T, Hörl D, Chen W, Durante M, Taucher-Scholz G, Leonhardt H, Cardoso MC (2017) Identification of the elementary structural units of the DNA damage response. Nat Commun 8:15760
Lopez Perez R, Best G, Nicolay NH, Greubel C, Rossberger S, Reindl J, Dollinger G, Weber KJ, Cremer C, Huber PE (2016) Superresolution light microscopy shows nanostructure of carbon ion radiation-induced DNA double-strand break repair foci. FASEB J 30:2767–2776
Sisario D, Memmel S, Doose S, Neubauer J, Zimmermann H, Flentje M, Djuzenova CS, Sauer M, Sukhorukov V (2018) Nanostructure of DNA repair foci revealed by superresolution microscopy. FASEB J 32:6469–6477
Mladenov E, Kalev P, Anachkova B (2009) The complexity of double-strand break ends is a factor in the repair pathway choice. Radiat Res 171:397–404
Sheen MR, Kim SW, Jung JY, Ahn JY, Rhee JG, Kwon HM, Woo SK (2006) Mre11-Rad50-Nbs1 complex is activated by hypertonicity. Am J Physiol Ren Physiol 291:F1014–F1020
van de Linde S, Löschberger A, Klein T, Heidbreder M, Wolter S, Heilemann M, Sauer M (2011) Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nat Protoc 6:991–1009
Wolter S, Löschberger A, Holm T, Aufmkolk S, Devauballe MC, van de Linde S, Sauer M (2012) rapidSTORM: accurate, fast open-source software for localization microscopy. Nat Methods 9:1040–1041
Levet F, Hosy E, Kechkar A, Butler C, Beghin A, Choquet D, Sibarita JB (2015) SR-Tesseler: a method to segment and quantify localization-based super-resolution microscopy data. Nat Methods 12:1065–1071
Aurenhammer F (1991) Voronoi diagrams—a survey of a fundamental geometric data structure. ACM Comput Surv 23:345–405
Chamma I, Levet F, Sibarita JB, Sainlos M, Thoumine O (2016) Nanoscale organization of synaptic adhesion proteins revealed by single-molecule localization microscopy. Neurophotonics 3:041810. https://doi.org/10.1117/1.NPh.3.4.041810
Hadipour-Lakmehsari S, Driouchi A, Lee SH, Kuzmanov U, Callaghan NI, Heximer SP, Simmons CA, Yip CM, Gramolini AO (2019) Nanoscale reorganization of sarcoplasmic reticulum in pressure-overload cardiac hypertrophy visualized by dSTORM. Sci Rep 9:7867. https://doi.org/10.1038/s41598-019-44331-y
Martinière A, Fiche JB, Smokvarska M, Mari S, Alcon C, Dumont X, Hematy K, Jaillais Y, Nollmann M, Maurel C (2019) Osmotic stress activates two reactive oxygen species pathways with distinct effects on protein nanodomains and diffusion. Plant Physiol 179:1581–1593
Pereira PM, Albrecht D, Culley S, Jacobs C, Marsh M, Mercer J, Henriques R (2019) Fix your membrane receptor imaging: actin cytoskeleton and CD4 membrane organization disruption by chemical fixation. Front Immunol 10:675. https://doi.org/10.3389/fimmu.2019.00675
Costes SV, Chiolo I, Pluth JM, Barcellos-Hoff MH, Jakob B (2010) Spatiotemporal characterization of ionizing radiation induced DNA damage foci and their relation to chromatin organization. Mutat Res 704:78–87
de Berg M, van Kreveld M, van Overmars M, Schwarzkopf O (2000) Computational geometry: algorithms and applications. Springer, Berlin, pp 2–8
Braden B (1986) The surveyor’s area formula. Coll Math J 17:326–337
Burma S, Chen B, Murphy M, Kurimasa A, Chen D (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276:42462–42467
Solier S, Sordet O, Kohn KW, Pommier Y (2009) Death receptor-induced activation of the chk2- and histone H2AX-associated DNA damage response pathway. Mol Cell Biol 29:68–82
de Feraudy S, Revet I, Bezrookove V, Feeney L, Cleaver JE (2010) A minority of foci or pan-nuclear apoptotic staining of gammaH2AX in the S phase after UV damage contain DNA double-strand breaks. Proc Natl Acad Sci USA 107:6870–6875. https://doi.org/10.1073/pnas.1002175107
Meyer B, Voss KO, Tobias F, Jakob B, Durante M, Taucher-Scholz G (2013) Clustered DNA damage induces pannuclear H2AX phosphorylation mediated by ATM and DNA-PK. Nucleic Acids Res 41:6109–6118
Mungunsukh O, Griffin AJ, Lee YH, Day RM (2010) Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 298:L696–L703
Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG (2009) Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 458:591–596
Dong Y, Xiong M, Duan L, Liu Z, Niu T, Luo Y, Wu X, Xu C, Lu C (2014) H2AX phosphorylation regulated by p38 is involved in Bim expression and apoptosis in chronic myelogenous leukemia cells induced by imatinib. Apoptosis 19:1281–1292
Costes SV, Boisière A, Ravani S, Romano R, Parvin B, Barcellos-Hoff MH (2006) Imaging features that discriminate between foci induced by high- and low-LET radiation in human fibroblasts. Radiat Res 165:505–515
McManus KJ, Hendzel MJ (2005) ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell 16:5013–5025
Pron G, Mahrour N, Orlowski S, Tounekti O, Poddevin B, Belehradek J Jr, Mir LM (1999) Internalisation of the bleomycin molecules responsible for bleomycin toxicity: a receptor-mediated endocytosis mechanism. Biochem Pharmacol 57:45–56
Chen J, Chen Y, He Q (2012) Action of bleomycin is affected by bleomycin hydrolase but not by caveolin-1. Int J Oncol 41:2245–2252
Olive PL, Banath JP (1993) Detection of DNA double-strand breaks through the cell cycle after exposure to X-rays, bleomycin, etoposide and 125IdUrd. Int J Radiat Biol 64:349–358
Burger RM, Peisach J, Horwitz SB (1981) Activated bleomycin: a transient complex of drug, iron, and oxygen that degrades DNA. J Biol Chem 256:11636–11644
Grigaravicius P, Rapp A, Greulich KO (2009) A direct view by immunofluorescent comet assay (IFCA) of DNA damage induced by nicking and cutting enzymes, ionizing 137Cs radiation, UV-A laser microbeam irradiation and the radiomimetic drug bleomycin. Mutagenesis 24:191–197
Phillips JE, Corces VG (2009) CTCF: master weaver of the genome. Cell 137:1194–1211
Szilard RK, Jacques PE, Laramée L, Cheng B, Galicia S, Bataille AR, Yeung M, Mendez M, Bergeron M, Robert F et al (2010) Systematic identification of fragile sites via genome-wide location analysis of gamma-H2AX. Nat Struct Mol Biol 17:299–305
Seo J, Kim SC, Lee HS, Kim JK, Shon HJ, Salleh NL, Desai KV, Lee JH, Kang ES, Kim JS, Choi JK (2012) Genome-wide profiles of H2AX and γ-H2AX differentiate endogenous and exogenous DNA damage hotspots in human cells. Nucleic Acid Res 40:5965–5974
Nouspikel T, Hanawalt PC (2002) DNA repair in terminally differentiated cells. DNA Repair 1:59–75
Turinetto V, Giachino C (2015) Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res 43:2489–2498
Hausmann M, Wagner E, Lee JH, Schrock G, Schaufler W, Krufczik M, Papenfuß F, Port M, Bestvater F, Scherthan H (2018) Super-resolution localization microscopy of radiation-induced histone H2AX-phosphorylation in relation to H3K9-trimethylation in HeLa cells. Nanoscale 10:4320–4331
Blackford AN, Jackson SP (2017) ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell 66:801–817
Salzano M, Sanz-García M, Monsalve DM, Moura DS, Lazo PA (2015) VRK1 chromatin kinase phosphorylates H2AX and is required for foci formation induced by DNA damage. Epigenetics 10:373–383
Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J (2005) gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell 20:801–809
Nakada S, Chen GI, Gingras AC, Durocher D (2008) PP4 is a gamma H2AX phosphatase required for recovery from the DNA damage checkpoint. EMBO Rep 9:1019–1026
Svetlova M, Solovjeva L, Nishi K, Nazarov I, Siino J, Tomilin N (2007) Elimination of radiation-induced gamma-H2AX foci in mammalian nucleus can occur by histone exchange. Biochem Biophys Res Commun 358:650–654
Jung EJ, Kim CW, Kim DR (2008) Cytosolic accumulation of gammaH2AX is associated with tropomyosin-related kinase A-induced cell death in U2OS cells. Exp Mol Med 40:276–285
Acknowledgements
We wish to express our gratitude to Markus Sauer for the invitation to perform dSTORM experiments at the Department of Biotechnology and Biophysics (University of Würzburg) as well as to Pablo Mateos-Gil, Sebastian Letschert and Fabian Zwettler for training and advice in dSTORM methodology (PL). We are also indebted to ANII (National Agency for Research and Innovation, Uruguay) for PhD Scholarship (POS_NAC_2014_1_102214) to PL as well as research support to LL-H and GF, and to PEDECIBA (Program for the Development of Basic Sciences, Uruguay). Research in SCIAN-Lab is funded by the Chilean Millennium Scientific Initiative P09-015-F to IC, JJ-W, SH; FONDECYT 11170475 to IC; FONDECYT 1181823 to IC, SH; FONDECYT 1161274, FONDECYT Ring Initiative ACT-1402, DAAD 57220037 and 57168868, CORFO 16CTTS-66390 to SH; CONICYT PhD Scholarship to JJ-W.
Author information
Authors and Affiliations
Contributions
PL: conceived and designed studies, performed research, analyzed data and wrote the draft manuscript. JJ-W co-wrote data analysis section in Materials and Methods and revised the manuscript. JJ-W, IC and SH developed the scripts for processing the SR-Tesseler files and contributed to data analysis. LL-H: contributed to design experiments, data interpretation and revised the manuscript. GF: revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2020_3809_MOESM1_ESM.tif
Supplementary file1 (TIF 10820 kb) Supplemental Fig. 1 Selection of the density factor threshold (α) to segment γH2AX foci regions from control and BLEO20 nuclei. In each case, the visual inspection of segmentation results was considered to set an optimal α value. Results are exemplified in a BLEO20 nucleus (a–i). From the localization file image (a), output images harboring the obtained segmented γH2AX foci regions after increasing values of α (0.1–10) are shown (b–i). The corresponding localizations associated, in each case, to the segmented γH2AX foci regions after applying the distinct α are depicted in blue. The nuclear ROI (r1) is outlined by the red line. Bar: 3 µm. As seen on the image, α values ≤ 0.25 overestimated the number of nuclear areas assigned as foci (b, c), while applying α values within the range [0.5, 2] (d–f) matched the foci observed in the original image (a). The use of α ≥ 3 resulted in a growing number of foci not being segmented (g–i), since α values became so demanding that most localization belonging to γH2AX foci regions were incorrectly linked to non-foci areas. In BLEO20 nuclei, α = 1.0 was the parameter value that best equaled the obtained foci segmentation with γH2AX foci regions. Since the mean localization density in the entire nucleus was lower in controls (307 localizations/μm2) than in BLEO-treated nuclei (620 localizations/μm2) a higher α value was needed in these cells to achieve a proper segmentation. α = 2.0 was found the value that best matched foci segmentation with endogenous γH2AX foci in control cells
11010_2020_3809_MOESM2_ESM.tif
Supplementary file2 (TIF 13890 kb) Supplemental Fig. 2 Adjustment of distance parameter d. A Python script was implemented to calculate extended convex hull polygons for each γH2AX focus, parameterized by a distance d, with the purpose of producing two new localization files from each original image (control and BLEO20 nuclei with foci and non-foci localizations) (Fig. 2). Since the parameters for foci segmentation provided in SR-Tesseler led to convex hull polygons (foci regions) with neighboring localizations that could not be included by any set of parameter values, a post-processing step was implemented within the Python script to include these localizations. d is an extra border distance from each convex hull that defines a neighborhood to include localizations from outside the convex hull polygons, reassigning them as part of the segmented foci regions. a–l Output images of a BLEO20 nucleus obtained with increasing values of d (0–1000 nm). Sets of localizations linked to non-foci areas (a, b, e, f, i, j) and the corresponding remaining localizations associated to γH2AX foci (c, d, g, h, k, l) are depicted. The nuclear ROI (r1) is outlined by the red line. Bar: 3 µm. As shown in a, using only the convex hull polygons with no additional distance (d = 0) for each object (γH2AX focus) resulted in a halo of points (localizations) surrounding each substracted γH2AX focus. d = 20 yielded a modest impact on the halos disappearance (b), while d = 50 nm was found to successfully remove them (e), reassigning halo localizations to the pooled foci areas (g). Larger neighborhoods (d ≥ 200 nm) resulted in oversegmentation, with localizations well outside γH2AX foci allocated to foci regions (h–l)
11010_2020_3809_MOESM3_ESM.tif
Supplementary file3 (TIF 228251 kb) Supplemental Fig. 3 dSTORM super-resolution imaging of γH2AX nuclear signal under distinct conditions of BLEO-exposure and post-damage recovery time. Cells were untreated (Control) or exposed to different doses of BLEO: 5 µg/mL (BLEO5), 20 µg/mL (BLEO20), 80 µg/mL (BLEO80) and 160 µg/mL (BLEO160) for 45 min before fixation (PFA 3.7%). In addition, cells exposed to BLEO20 for 45 min were allowed to recover in fresh culture medium for different post-damage times (0.5 h, 1 h and 2 h) before being fixed and immunolabeled. a and b Average foci number per cell (mean ± standard error) in relation to BLEO dose (no recovery time) (a) or in cultures treated with BLEO20 and subjected to different post-damage recovery times (0–2 h) (b). For each condition 11 < n < 32 nuclei, coming from at least two independent experiments, were analyzed (**p < 0.01, ***p < 0.001 with respect to control by one-way ANOVA with Bonferroni correction). An appropriate level of γH2AX foci induction was obtained over the entire dose range (5–160 µg/mL) (a). An increase in foci number was detected only for BLEO160 in comparison with the other doses of BLEO (p < 0.05). Otherwise, a similar outcome was found between cultures exposed to BLEO20 but recovered at different post-damage times (0–2 h) (b). c–m Representative dSTORM images of HeLa nuclei for each condition are shown. Bar: 3 µm. Immunolabeling was performed either with Alexa Fluor 647 (c–k) or Alexa Fluor 532 (l, m) secondary antibodies. Upper left insets: corresponding γH2AX images for each nucleus by standard wide-field fluorescence microscopy. In all cases γH2AX foci were observed to be composed of smaller nanometric subunits when samples were incubated with the primary anti-γH2AX antibody (c–l)
11010_2020_3809_MOESM4_ESM.tif
Supplementary file4 (TIF 55769 kb) Supplemental Fig. 4 Preclusion of γH2AX foci formation was maximized under simultaneous treatment with ATM, ATR and DNA-PK inhibitors. HeLa cells were pretreated for 1 h with 20 µM of ATM, ATR and/or DNA-PK inhibitors (denoted ATMi, ATRi and DNA-PKi, respectively), either alone or in combinations, and then co-exposed for 45 min to BLEO20 and the inhibitor(s) before being fixed in 3.7% PFA and immunostained for γH2AX (Alexa Fluor 532 or Alexa Fluor 647). In addition, untreated cell cultures (control), exposed only to BLEO20 or to the solvent of inhibitors (vehicle control; 1.2% DMSO) were considered. a Foci number per cell (mean ± standard error) for each condition. 10–20 CLSM z-stacks having several nuclei per field, from three independent experiments, were analyzed. 250–650 cells were considered in each case. A diminution of foci number was detected only in BLEO-treated cultures exposed either to ATMi alone or in combination with the other inhibitors when compared to BLEO20 or BLEO20 cultures exposed to ATRi and/or DNA-PKi (**p < 0.01 respect to BLEO20 by one-way ANOVA with Bonferroni correction). b–j Representative slices from cultures exposed to each condition. γH2AX immunostaining is shown in grey. Bar: 15 µm. Occasionally, γH2AX pan-nuclear cells were detected in BLEO-treatment cultures even under simultaneous treatment with the three kinase inhibitors
11010_2020_3809_MOESM5_ESM.tif
Supplementary file5 (TIF 4314 kb) Supplemental Fig. 5 Distribution of γH2AX foci areas for control and BLEO20 nuclei. The histograms of the segmented object areas (μm2) obtained by combining SR-Tesseler with a custom script (Fig. 2) are shown. Altogether, n = 135 (control) and n = 638 (BLEO20) γH2AX foci coming from n = 11 (control) and n = 21 (BLEO20) cells were considered (data from three independent experiments). For each condition, mean foci areas (± standard error) are depicted on the plot. As evidenced by the histogram, BLEO-induced γH2AX foci exhibited a broad range of size, covering all histogram bins. Conversely, endogenous foci in controls were mainly restricted to areas among 0.025 µm2 (the minimum area considered) and 0.3 µm2. On average, BLEO-induced foci areas were approximately two times bigger than endogenous foci areas
11010_2020_3809_MOESM6_ESM.tif
Supplementary file6 (TIF 4318 kb) Supplemental Fig. 6 Presence of γH2AX signal outside nuclei. Images from localization files of a Control, b BLEO20, c BLEO20 + 3i and d Ab control nuclei in SR-Tesseler are shown. Nuclear ROI are outlined by red lines. Bar: 3 µm. As seen on images, in all conditions γH2AX signals were also observed external to nuclear ROI (that is, probably in cytoplasmic areas). By drawing ROI in these regions (not shown) we verified that this signal could be well segmented with the same parameters as the scattered nuclear γH2AX mark. Nevertheless, signal densities were not exhaustively calculated since cytoplasm boundaries could not be precisely defined. In any case, for all conditions the extranuclear density was considerably lower than the nuclear γH2AX density inside ROI (rough estimation)
Rights and permissions
About this article
Cite this article
Liddle, P., Jara-Wilde, J., Lafon-Hughes, L. et al. dSTORM microscopy evidences in HeLa cells clustered and scattered γH2AX nanofoci sensitive to ATM, DNA-PK, and ATR kinase inhibitors. Mol Cell Biochem 473, 77–91 (2020). https://doi.org/10.1007/s11010-020-03809-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03809-4