Abstract
Diabetic nephropathy and cardiomyopathy are two major causes of mortality among patients with diabetes mellitus (DM). Since current diabetic medications are associated with various side effects, the naturally occurring plant-derived compounds are in demand. Bioflavonoids originating from vegetables and medicinal plants have beneficial effects on diabetes by improving glycemic control, lipid metabolism, and anti-oxidant status. The present study is focused on the effect of rutin against alloxan induced diabetic nephropathy and cardiomyopathy. Male albino Wistar rats were divided into four groups, each of six rats. Group I control rats received 0.9% saline as a single dose intraperitoneally. Group II rats were induced diabetes with a single dose of alloxan monohydrate (150 mg/kg body weight in 0.9% saline) intraperitoneally. Group III rats received 0.28 M of NH4Cl in drinking water for 3 days for the experimental induction of metabolic acidosis. Group IV rats were injected with a single dose of alloxan monohydrate (150 mg/kg bodyweight) and administered rutin hydrate (100 mg/kg) for a period of 4 weeks by oral gavage. Administration of rutin prevented urinary ketone body formation and decreased serum creatinine and urea levels in alloxan induced diabetic rats. Rutin supplementation reduced the levels of serum triglycerides and cholesterol in diabetic rats. Gene expression profiling of metabolic acidosis related genes (AQP2, AQP3 and V2R) and also histopathological results demonstrated the protective effect of rutin against diabetic ketoacidodis and fibrosis. The results of the present study revealed rutin administration prevents the progression of diabetic nephropathy and cardiomyopathy through amelioration of fibrosis and metabolic acidosis.
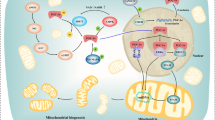
Graphic abstract






Similar content being viewed by others
References
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE (2017) IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50
Cho N, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, OhlroggeMalanda AWB (2018) IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281
Sharma D, Bhattacharya P, Kalia K, Tiwari V (2017) Diabetic nephropathy: new insights into established therapeutic paradigms and novel molecular targets. Diabetes Res Clin Pract 128:91–108
Lim AK (2014) Diabetic nephropathy–complications and treatment. Int J Nephrol Renovasc Dis 7:361–381
Parchwani DN, Upadhyah AA (2012) Diabetic nephropathy: progression and pathophysiology. Int J Med Sci Public Health 1:59–70
Liu Q, Wang S, Cai L (2014) Diabetic cardiomyopathy and its mechanisms: role of oxidative stress and damage. J Diabetes Investig 5:623–634
London GM (2002) Left ventricular alterations and end-stage renal disease. Nephrol Dial Transplant 17:29–36
Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE (2009) The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol 53:2129–2140
Kitabchi AE, Nyenw EA (2006) Hyperglycemic crises in diabetes mellitus: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin 35:725–751
Souto G, Donapetry C, Calvino J, Adeva MM (2011) Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab Syndr Relat Disord 9(4):247–253
Kraut JA, Madias NE (2010) Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol 6:274–285
Singh P, Carraher C, Schwarzbauer JE (2010) Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26:397–419
Tammaro G, Zacchia M, Zona E, Zacchia E, Capasso G (2018) Acute and chronic effects of metabolic acidosis on renal function and structure. J Nephrol 31:551–559
Kim Y, Park CW (2017) New therapeutic agents in diabetic nephropathy. Korean J Intern Med 32:11–25
Dobre M, Rahman M, Hostette TH (2015) Current status of bicarbonate in CKD. J Am Soc Nephrol 26:515–523
Breyer MD, Susztak K (2016) The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov 15:568–588
Gomes IB, Porto ML, Santos MCL, Campagnaro BP, Pereira TM, Meyrelles SS, Vasquez EC (2014) Renoprotective, anti-oxidative and anti-apoptotic effects of oral low-dose quercetin in the C57BL/6J model of diabetic nephropathy. Lipids Health Dis 13:184–187
Wang YB, Ge ZM, Kang WQ, Lian ZX, Yao J, Zhou CY (2015) Rutin alleviates diabetic cardiomyopathy in a rat model of type 2 diabetes. Exp Ther Med 9:451–455
Han CS, Liu K, Zhang N, Li SW, Gao HC (2017) Rutin suppresses high glucose-induced ACTA2 and p38 protein expression in diabetic nephropathy. Exp Ther Med 14:181–186
Penders J, Fiers T, Giri M, Wuyts B, Ysewyn L, Delanghe JR (2005) Quantitative measurement of ketone bodies in urine using reflectometry. Clin Chem Lab Med 43:724–729
Liu JE, Robbins DC, Palmieri V, Bella JN, Roman MJ, Fabsitz R, Howard BV, Welty TK, Lee ET, Devereux RB (2003) Association of albuminuria with systolic and diastolic left ventricular dysfunction in type 2 diabetes: the Strong Heart Study. J Am Coll Cardiol 41:2022–2028
Ma TK, Kam KK, Yan BP, Lam YY (2010) Renin–angiotensin–aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol 160:1273–1292
Abdel Aziz MA, Badary DM, Hussein MRA (2017) Renal damage following Alloxan-induced diabetes is associated with generation of reactive oxygen species, alterations of p53, TGF-β1, and extracellular matrix metalloproteinases in rats. Cell Biol Int 41:525–533
Kim MJ, Ha BJ (2014) Antihyperglycemic and antihyperlipidemic effects of fermented Rhynchosia nulubilis in alloxan-induced diabetic rats. Toxicol Res 29:15–19
Bako HY, Mohammad JS, Wazir PM, Bulus T, Gwarzo MY, Zubairu MM (2014) Lipid profile of alloxan-induced diabetic wistar rats treated with methanolic extract of Adansonia digitata fruit pulp. Sci World J 9:19–24
Ramakrishnan A, Vijayakumar N, Renuka M (2016) Naringin regulates glutamate-nitric oxide cGMP pathway in ammonium chloride induced neurotoxicity. Biomed Pharmacother 84:1717–1726
Ganeshpurkar A, Saluja AK (2017) The pharmacological potential of rutin. Saudi Pharm J 25:149–164
Kraut JA, Madias NE (2007) Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2:162–174
He J, Yang B (2019) Aquaporins in renal diseases. Int J Mol Sci 20(2):366
Nowik M, Kampik NB, Mihailova M, Eladari D, Wagner CA (2010) Induction of metabolic acidosis with ammonium chloride (NH4Cl) in mice and rats–species differences and technical considerations. Cell Physiol Biochem 26:1059–1072
Amlal H, Sheriff S, Soleimani M (2004) Upregulation of collecting duct aquaporin-2 by metabolic acidosis: role of vasopressin. Am J Physiol Cell Physiol 286:1019–1030
Dhondup T, Qian Q (2017) Electrolyte and acid-base disorders in chronic kidney disease and end-stage kidney failure. Blood Purif 43:179–188
Hsieh CL, Peng CC, Chen KC, Peng RY (2013) Rutin (quercetin rutinoside) induced protein-energy malnutrition in chronic kidney disease, but quercetin acted beneficially. J Agric Food Chem 61:7258–7267
Diwan V, Brown L, Gobe GC (2017) The flavonoid rutin improves kidney and heart structure and function in an adenine-induced rat model of chronic kidney disease. J Funct Foods 33:85–93
Caglayan C, Kandemir FM, Yildirim S, Kucukler S, Eser G (2019) Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J Trace Elem Med Biol 54:69–78
Acknowledgments
This work was supported by DBT-IPLS, CSIR-SRF fellowship, New Delhi, India. The authors also thank UGC-CEGS, UGC-CAS, UGC-NRCBS, DST-FIST, and DST-PURSE program for the central instrumentation facility at SBS, MKU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The experimental procedure was approved by the Internal Research and Review Board, Ethical Clearance, Biosafety and Animal Welfare Committee of Madurai Kamaraj University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ganesan, D., Albert, A., Paul, E. et al. Rutin ameliorates metabolic acidosis and fibrosis in alloxan induced diabetic nephropathy and cardiomyopathy in experimental rats. Mol Cell Biochem 471, 41–50 (2020). https://doi.org/10.1007/s11010-020-03758-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03758-y