Abstract
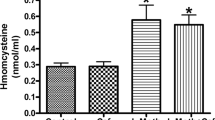
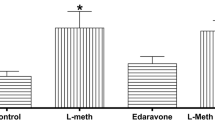
The aim of this study was to evaluate the effects of atorvastatin and simvastatin on behavioral manifestations that followed hyperhomocysteinemia induced by special dietary protocols enriched in methionine and deficient in B vitamins (B6, B9, B12) by means of alterations in anxiety levels in rats. Simultaneously, we investigated the alterations of oxidative stress markers in rat hippocampus induced by applied dietary protocols. Furthermore, considering the well-known antioxidant properties of statins, we attempted to assess their impact on major markers of oxidative stress and their possible beneficial role on anxiety-like behavior effect in rats. The 4-week-old male Wistar albino rats were divided (eight per group) according to basic dietary protocols: standard chow, methionine-enriched, and methionine-enriched vitamins B (B6, B9, B12) deficient. Each dietary protocol (30 days) included groups with atorvastatin (3 mg/kg/day i.p.) and simvastatin (5 mg/kg/day i.p.). The behavioral testing was performed in the open field and elevated plus maze tests. Parameters of oxidative stress (index of lipid peroxidation, superoxide dismutase, catalase activity, glutathione) were determined in hippocampal tissue samples following decapitation after anesthesia. Methionine-load dietary protocols induced increased oxidative stress in rat hippocampus, which was accompanied by anxiogenic behavioral manifestations. The methionine-enriched diet with restricted vitamins B intake induced more pronounced anxiogenic effect, as well as increased oxidative stress compared to the methionine-load diet with normal vitamins B content. Simultaneous administration of statins showed beneficial effects by means of both decreased parameters of oxidative stress and attenuation of anxiety. The results obtained with simvastatin were more convincible compared to atorvastatin.








Similar content being viewed by others
Abbreviations
- Met:
-
Methionine
- SAM:
-
S-adenosylmethionine
- SAH:
-
S-adenosylhomocysteine
- Hcy:
-
Homocysteine
- MS:
-
Methionine synthase
- GSH:
-
Glutathione
- SH:
-
Sulfhydril
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- OF:
-
Open field
- EPM:
-
Elevated plus maze
- TBARS:
-
Thiobarbituric acid reactive substance
- MDA:
-
Malondialdehyde
References
Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A, Červeňová T, Halašova E, Lehotský J (2016) The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci 17(10):1733
Lu SC (2000) S-Adenosylmethionine. Int J Biochem Cell Biol 32:391–395
Petras M, Tatarkova Z, Kovalska M, Mokra D, Dobrota D, Lehotsky J, Drgova A (2014) Hyperhomocysteinemia as a risk factor for the neuronal system disorders. J Physio Pharmacol 65(1):15–23
Obeid R, Herrmann W (2006) Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 580:2994–3005
Reynolds EH, Carney MW, Toone BK (1984) Methylation and mood. Lancet 2:196–198
Türksoy N, Bilici R, Yalçıner A, Ozdemir Y, Ornek I, Tufan AE, Kara A (2014) Vitamin B12, folate, and homocysteine levels in patients with obsessive-compulsive disorder. Neuropsychiatr Dis Treat 10:1671–1675
Lehmann M, Gottfries C, Regland G B (1999) Identification of Cognitive impairment in the elderly: Homocysteine is an farly marker. Dement Geriatr Cogn Disord 10:12–20
Kahler SG, Fahey MC (2003) Metabolic disorders and mental retardation. Am J Med Genet Part 117:31–41
Chamberlin ME, Ubagai T, Mudd SH, Wilson WG, Leonard JV, Chou JY (1996) Demyelination of the brain is associated with methionine adenosyltransferase I/III deficiency. JCI 98(4):1021–1027
Oulhaj A, Refsum H, Beaumont H, Williams J, King E, Jacoby R, Smith AD (2010) Homocysteine as a predictor of cognitive decline in Alzheimer’s disease. Int J Geriatr Psychiatry 25:82–90
Blandini F, Fancellu R, Martignoni E, Mangiagalli A, Pacchetti C, Samuele A, Nappi G (2001) Plasma homocysteine and l-dopa metabolism in patients with Parkinson disease. Clin Chem 47(6):1102–1104
Hankey GJ, Eikelboom JW (2001) Homocysteine and stroke. Curr Opin Neurol 14(1):95–102
Obeid R, Mc Caddon A, Herrmann W (2007) The role of hyperhomocysteinemia and B vitamin deficiency in neurological and psychiatric diseases. Clin Chem Lab Med 45(12):1590–1606
Moustafa AA, Hewedi DH, Eissa AM, Frydecka D, Misiak B (2014) Homocysteine levels in schizophrenia and affective disorders—focus on cognition. Front Behav Neurosci 8:343
Gu P, DeFina LF, Leonard D, John S, Weiner MF, Brown ES (2012) Relationship between serum homocysteine levels and depressive symptoms: the Cooper Center Longitudinal Study. J Clin Psychiatr 73:691–695
Folstein M, Liu T, Peter I, Buell J, Arsenault L, Scott T et al (2007) The homocysteine hypothesis of depression. Am J Psychiatr 164:861–867
Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MB (2002) Vitamin B12, folate, and homocysteine in depression: the Rotterdam study. Am J Psychiatr 159:2099–2101
Chung KH, Chiou HY, Chen YH (2017) Associations between serum homocysteine levels and anxiety and depression among children and adolescents in Taiwan. Sci Rep 7(1):8330
Atmaca M, Tezcan E, Kuloglu M, Kirtas O, Ustandag B (2005) Serum folate and homocysteine levels in patients with obsessive–compulsive disorder. Psychiatry Clin Neurosci 59(5):616–620
Levine J, Timinsky I, Vishne T et al (2008) Elevated serum homocysteine levels in male patients with PTSD. Depress Anxiety 25(11):154–157
Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS et al (2001) Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet 10:433–443
Jakubowski H, Perla-Kaján J, Finnell RH, Cabrera RM, Wang H, Gupta S, Kruger WD, Kraus JP, Shih DM (2009) Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. FASEB J 23:1721–1727
Parsons RB, Waring RH, Ramsden DB, Williams AC (1998) In vitro effect of the cysteine metabolites homocysteic acid, homocysteine and cysteic acid upon human neuronal cell lines. Neurotoxicology 19:599–603
Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP (2000) Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci 20:6920–6926
Zou CG, Banerjee R (2005) Homocysteine and redox signaling. Antioxid Redox Signal 7:547–559
Perna AF, Ingrosso D, De Santo NG (2003) Homocysteine and oxidative stress. Amino Acids 25:409–417
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97(6):1634–1658
Weber GF (1994) The pathophysiology of reactive oxygen intermediates in the central nervous system. Med Hypotheses 43(4):223–230
Jara-Prado A, Ortega-Vazquez A, Martinez-Ruano L, Rios C, Santamaria A (2003) Homocysteine-induced brain lipid peroxidation: effects of NMDA receptor blockade, antioxidant treatment, and nitric oxide synthase inhibition. Neurotox Res 5(4):237–243
Lebel C (1991) Oxygen radicals: Common mediators of neurotoxicity. Neurotox Teratol 13:341–346
Herken H, Akyol O, Yilmaz HR, Tutkun H, Savas HA, Ozen ME, Kalenderoglu A, Gulec M (2006) Nitric oxide, adenosine deaminase, xanthine oxidase and superoxide dismutase in patients with panic disorder: alterations by antidepressant treatment. Hum Psychopharmacol 21:53–59
Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, Kap O, Yumru M, Savas HA, Akyol O (2007) Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res 38:247–252
Ersan S, Bakir S, Erdal Ersan E, Dogan O (2006) Examination of free radical metabolism and antioxidant defence system elements in patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatr 30:1039–1042
Kodydkova J, Vavrova L, Zeman M, Jirak R, Macasek J, Stankova B, Tvrzicka E, Zak A (2009) Antioxidative enzymes and increased oxidative stress in depressive women. Clin Biochem 42:1368–1374
Ersoy MA, Selek S, Celik H, Erel O, Kaya MC, Savas HA, Herken H (2008) Role of oxidative and antioxidative parameters in etiopathogenesis and prognosis of panic disorder. Int J Neurosci 118:1025–1037
Selek S, Herken H, Bulut M, Ceylan MF, Celik H, Savas HA, Erel O (2008) Oxidative imbalance in obsessive compulsive disorder patients: a total evaluation of oxidant-antioxidant status. Prog Neuropsychopharmacol Biol Psychiatr 32:487–491
Atmaca M, Kuloglu M, Tezcan E, Ustundag B (2008) Antioxidant enzyme and malondialdehyde levels in patients with social phobia. Psychiatr Res 159:95–100
Galecki P, Szemraj J, Bienkiewicz M, Florkowski A, Galecka E (2009) Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacol Rep 61:436–447
Viggiano A, Viggiano E, Monda M, Ingrosso D, Perna AF, De Luca B (2012) Methionine-enriched diet decreases hippocampal antioxidant defences and impairs spontaneous behaviour and long term potentiation in rats. Brain Res 1471:66–74
Hrnčić D, Mikić J, Rašić-Marković A, Velimirović M, Stojković T, Obrenović R, Rankov-Petrović B, Šušić V, Djurić D, Petronijević N, Stanojlović O (2016) Anxiety-related behavior in hyperhomocysteinemia induced by methionine nutritional overload in rats: role of the brain oxidative stress. Can J Physiol Pharmacol 94(10):1074–1082
Hovatta I, Juhila J, Donner J (2010) Oxidative stress in anxiety and comorbid disorders. Neurosci Res 68(4):261–275
Rosic G, Joksimovic J, Selakovic D, Jakovljevic V, Živkovic V, Srejovic I, Djuric M, Djuric D (2018) The beneficial effects of sulfur-containing amino acids on cisplatin-induced cardiotoxicity and neurotoxicity in rodents. Curr Med Chem 25(3):391–403
Lakhan V (2010) Nutritional and herbal supplements for anxiety and anxiety-related disorders: systematic review. Nutr J 9:42
Vignes M, Maurice T, Lanté F, Nedjar M, Thethi K, Guiramand J, Récasens M (2006) Anxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG). Brain Res 1110:102–115
McFarlane SI, Muniyappa R, Francisco R, Sowers JR (2002) Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab 87(4):1451–1458
Murrow JR, Sher S, Ali S, Uphoff I, Patel R, Porkert M et al (2012) The differential effect of statins on oxidative stress and endothelial function: atorvastatin versus pravastatin. J Clin Lipidol 6(1):42–49
Van der Most PJ, Dolga AM, Nijholt IM, Luiten PGM, Eisel ULM (2009) Statins: mechanisms of neuroprotection. Prog Neurobiol 88(1):64–75
Mohammadi MT, Amini R, Jahanbakhsh Z, Shekarforoush S (2013) Effects of atorvastatin on the hypertension-induced oxidative stress in the rat brain. IBJ 17(3):152–157
ElBatsh MM (2015) Antidepressant-like effect of simvastatin in diabetic rats. Can J Physiol Pharmacol 93(8):649–656
Lin PY, Chang AY, Lin TK (2014) Simvastatin treatment exerts antidepressant-like effect in rats exposed to chronic mild stress. Pharmacol Biochem Behav 124:174–179
Can ÖD, Ulupınar E, Özkay ÜD, Yegin B, Öztürk Y (2012) The effect of simvastatin treatment on behavioral parameters, cognitive performance, and hippocampal morphology in rats fed a standard or a high-fat diet. Behav Pharmacol 23:582–592
Citraro R, Chimirri S, Aiello R, Gallelli L, Trimboli F, Britti D, De Sarro G, Russo E (2014) Protective effects of some statins on epileptogenesis and depressive-like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia 55(8):1284–1291
Anupama GM, Shrishail HV, Shashikant T (2013) Evaluation of antidepressant activity of simvastatin, lovastatin and atorvastatin in male swiss mice - an experimental study. Int J Drug Dev Res 5(2):102–108
Bjelland I, Tell G, Vollset S, Refsuem H, Ueland P (2003) Folate, vitamin B12, homocysteine, and the MTHFR 677CўT polymorphism in anxiety and depression, the Hordaland Homocysteine Study. Arch Gen Psychiatr 60(6):618–626
Sodha NR, Boodhwani M, Ramlawi B, Clements RT, Mieno S, Feng J, Xu SH, Bianchi S, Sellke FW (2008) Atorvastatin increases myocardial indices of oxidative stress in a porcine model of hypercholesterolemia and chronic ischemia. J Card Surg 23(4):312–320
Parle M, Singh N (2007) Reversal of memory deficits by atorvastatin and simvastatin in rats. Yakugaku Zasshi 127(7):1125–1137
Prut L, Belzung C (2003) The open field as a paradigm to measure the effect of drugs on anxiety-like behaviours: a review. Eur J Pharmacol 463(1–3):3–33
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14(3):149–167
Pellow S, File SE (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24(3):525–529
Selakovic D, Joksimovic J, Obradovic D, Milovanovic D, Djuric M, Rosic G (2016) The adverse effects of exercise and supraphysiological dose of testosterone-enanthate (TE) on exploratory activity in elevated plus maze (EPM) test—indications for using total exploratory activity (TEA) as a new parameter for exploratory activity estimation in EPM. Neuroendocrinol Lett 37(5):101–106
Li KW (2011) Neuroproteomics. Humana Press Springer, New York
Wohlenberg M, Almeida D, Bokowski L, Medeiros N, Agostini F, Funchal C, Dani C (2014) Antioxidant activity of grapevine leaf extracts against oxidative stress induced by carbon tetrachloride in cerebral cortex, hippocampus and cerebellum of rats. Antioxidants 3(2):200–211
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195(1):133–140
Ellman GL (1959) Tissue sulphydryl group. Arch Biochem Biophys 82:70–77
Lowry OH, Rosebrough NL, Farr AL, Randall RI (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Streck EL, Vieira PS, Wannmacher CM, Dutra-Filho CS, Wajner M, Wyse AT (2003) In vitro effect of homocysteine on some parameters of oxidative stress in rat hippocampus. Metab Brain Dis 18(2):147–154
Nikolić T (2017) The effects of hyperhomocysteinemia on myocardial function, coronary circulation and redox status of the isolated heart rat: role of hydroxymethyl glutaryl inhibitor coenzyme-A (HMG-COA) reductase. Dissertation, University of Kragujevac
Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD (2006) Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood 107(2):591–593
Ridker PM, Shih J, Cook TJ et al (2002) Plasma homocysteine concentration, statin therapy, and the risk of first acute coronary events. Circulation 105:1776–1779
Dierkes J, Luley C, Westphal S (2007) Effect of lipid-lowering and anti-hypertensive drugs on plasma homocysteine levels. Vasc Health Risk Manag 3(1):99–108
Jiang S, Chen Q, Venners SA, Zhong G, Hsu YH, Xing H, Wang X, Xu X (2013) Effect of simvastatin on plasma homocysteine levels and its modification by MTHFR C677T polymorphism in Chinese patients with primary hyperlipidemia. Cardiovasc Ther 31(4):27–33
Ludman A, Venugopal V, Yellon DM, Hausenloy DJ (2009) Statins and cardioprotection—more than just lipid lowering? Pharmacol Ther 122(1):30–43
Clarke AT, Johnson PC, Hall GC, Ford I, Mills PR (2016) High dose atorvastatin associated with increased risk of significant hepatotoxicity in comparison to simvastatin in UK GPRD cohort. PLoS ONE 11(3):e0151587
Selakovic D, Joksimovic J, Zaletel I, Puskas N, Matovic M, Rosic G (2017) The opposite effects of nandrolone decanoate and exercise on anxiety levels in rats may involve alterations in hippocampal parvalbumin-positive interneurons. PLoS ONE 12(12):e0189595
Hrnčić D, Rašić- Marković A, Mikić J, Demchuk G, Leković J, Šušić V, Macut D, Djurić D, Stanojlović O (2013) Anxiety-related behavior in adult rats after acute homocysteine thiolactone treatment. Clin Neurophysiol 124(7):14–15
Kilic FS, Ozatik Y, Kaygisiz B, Baydemir C, Erol K (2012) Acute antidepressant and anxiolytic effects of simvastatin and its mechanisms in rats. Neurosciences 17(1):39–43
Pemminati S, Nandini Colaco MB, Patchava D, Shivaprakash G, Sheetal Ullal D, Gopalakrishna HN, Rathnakar UP, Shenoy AK (2012) Role of statins in animal models of anxiety in Normo-cholesterolemic rats. J Pharm Res 5(7):3764–3766
Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM (2003) Long-term statin use and psychological well-being. J Am Coll Cardiol 42(4):690–697
Koladiya RU, Jaggi AS, Singh N, Sharma BK (2008) Ameliorative role of Atorvastatin and Pitavastatin in L-Methionine induced vascular dementia in rats. BMC Pharmacol 8:14
Liu W, Zhao Y, Zhang X, Ji J (2018) Simvastatin ameliorates cognitive impairments via inhibition of oxidative stress induced apoptosis of hippocampal cells through the ERK/AKT signaling pathway in a rat model of senile dementia. Mol Med Rep 17(1):1885–1892
Acknowledgements
This work was supported by the Faculty of Medical Sciences (JP 01/13), University of Kragujevac, Serbia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mijailovic, N., Selakovic, D., Joksimovic, J. et al. The anxiolytic effects of atorvastatin and simvastatin on dietary-induced increase in homocysteine levels in rats. Mol Cell Biochem 452, 199–217 (2019). https://doi.org/10.1007/s11010-018-3425-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3425-6