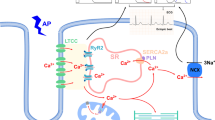
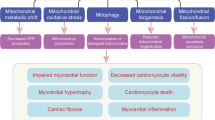
Abstract
Diabetic cardiomyopathy and heart failure have been recognized as the leading causes of mortality among diabetics. Diabetic cardiomyopathy has been characterized primarily by the manifestation of left ventricular dysfunction that is independent of coronary artery disease and hypertension among the patients affected by diabetes mellitus. A complex array of contributing factors including the hypertrophy of left ventricle, alterations of metabolism, microvascular pathology, insulin resistance, fibrosis, apoptotic cell death, and oxidative stress have been implicated in the pathogenesis of diabetic cardiomyopathy. Nevertheless, the exact mechanisms underlying the pathogenesis of diabetic cardiomyopathy are yet to be established. The critical involvement of multifarious factors including the vascular endothelial dysfunction, microangiopathy, reactive oxygen species (ROS), oxidative stress, mitochondrial dysfunction has been identified in the mechanism of pathogenesis of diabetic cardiomyopathy. Although it is difficult to establish how each factor contributes to disease, the involvement of ROS and mitochondrial dysfunction are emerging as front-runners in the mechanism of pathogenesis of diabetic cardiomyopathy. This review highlights the role of vascular endothelial dysfunction, ROS, oxidative stress, and mitochondriopathy in the pathogenesis of diabetic cardiomyopathy. Furthermore, the review emphasizes that the puzzle has to be solved to firmly establish the mitochondrial and/or ROS mechanism(s) by identifying their most critical molecular players involved at both spatial and temporal levels in diabetic cardiomyopathy as targets for specific and effective pharmacological/therapeutic interventions.




Similar content being viewed by others
References
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14
Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, Nichols G (2010) Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:293–301
Giacomelli F, Wiener J (1979) Primary myocardial disease in the diabetic mouse. An ultrastructural study. Lab Invest 40:460–473
Roda L, Patessio A, Neri V, Tisi C, Ferrari A, Ricci A (1980) Diabetic cardiomyopathy in preclinical phase. Polycardiographic and echocardiographic study (author’s transl). G Ital Cardiol 10:1299–1307
Scott RC (1984) Cardiomyopathy in diabetic patients. West J Med 140:610–612
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30:595–602
Liu JE, Palmieri V, Roman MJ, Bella JN, Fabsitz R, Howard BV, Welty TK, Lee ET, Devereux RB (2001) The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol 37:1943–1949
Kannel WB, Hjortland M, Castelli WP (1974) Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 34:29–34
Aronow WS, Ahn C (1999) Incidence of heart failure in 2,737 older persons with and without diabetes mellitus. Chest 115:867–868
Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB (2004) The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 27:1879–1884
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412
Garcia MJ, McNamara PM, Gordon T, Kannel WB (1974) Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes 23:105–111
Gaede P, Vedel P, Parving HH, Pedersen O (1999) Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 353:617–622
Poornima IG, Parikh P, Shannon RP (2006) Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 98:596–605
Raev DC (1994) Left ventricular function and specific diabetic complications in other target organs in young insulin-dependent diabetics: an echocardiographic study. Heart Vessels 9:121–128
Schannwell CM, Schoebel FC, Heggen S, Marx R, Perings C, Leschke M, Strauer BE (1999) Early decrease in diastolic function in young type I diabetic patients as an initial manifestation of diabetic cardiomyopathy. Z Kardiol 88:338–346
Maya L, Villarreal FJ (2010) Diagnostic approaches for diabetic cardiomyopathy and myocardial fibrosis. J Mol Cell Cardiol 48:524–529
Khouri SJ, Maly GT, Suh DD, Walsh TE (2004) A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr 17:290–297
Di Bonito P, Moio N, Cavuto L, Covino G, Murena E, Scilla C, Turco S, Capaldo B, Sibilio G (2005) Early detection of diabetic cardiomyopathy: usefulness of tissue Doppler imaging. Diabet Med 22:1720–1725
Boyer JK, Thanigaraj S, Schechtman KB, Perez JE (2004) Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 93:870–875
Saraiva RM, Duarte DM, Duarte MP, Martins AF, Poltronieri AV, Ferreira ME, Silva MC, Hohleuwerger R, Ellis A, Rachid MB, Monteiro CF, Kaiser SE (2005) Tissue Doppler imaging identifies asymptomatic normotensive diabetics with diastolic dysfunction and reduced exercise tolerance. Echocardiography 22:561–570
Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG (2001) Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 24:5–10
Raev DC (1994) Which left ventricular function is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care 17:633–639
Von Bibra H, Thrainsdottir IS, Hansen A, Dounis V, Malmberg K, Ryden L (2005) Tissue Doppler imaging for the detection and quantitation of myocardial dysfunction in patients with type 2 diabetes mellitus. Diab Vasc Dis Res 2:24–30
Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA (2001) Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 87:320–323
Henry RM, Paulus WJ, Kamp O, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD (2008) Deteriorating glucose tolerance status is associated with left ventricular dysfunction—the Hoorn Study. Neth J Med 66:110–117
Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ (2011) Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 306:856–863
Astorri E, Fiorina P, Contini GA, Albertini D, Magnati G, Astorri A, Lanfredini M (1997) Isolated and preclinical impairment of left ventricular filling in insulin-dependent and non-insulin-dependent diabetic patients. Clin Cardiol 20:536–540
Robillon JF, Sadoul JL, Jullien D, Morand P, Freychet P (1994) Abnormalities suggestive of cardiomyopathy in patients with type 2 diabetes of relatively short duration. Diabete Metab 20:473–480
Salazar J, Rivas A, Rodriguez M, Felipe J, Garcia MD, Bone J (1994) Left ventricular function determined by Doppler echocardiography in adolescents with type I (insulin-dependent) diabetes mellitus. Acta Cardiol 49:435–439
Dinh W, Lankisch M, Nickl W, Gies M, Scheyer D, Kramer F, Scheffold T, Krahns T, Sause A, Futh R (2011) Metabolic syndrome with or without diabetes contributes to left ventricular diastolic dysfunction. Acta Cardiol 66:167–174
Dinh W, Lankisch M, Nickl W, Scheyer D, Scheffold T, Kramer F, Krahn T, Klein RM, Barroso MC, Futh R (2010) Insulin resistance and glycemic abnormalities are associated with deterioration of left ventricular diastolic function: a cross-sectional study. Cardiovasc Diabetol 9:63
Futh R, Dinh W, Bansemir L, Ziegler G, Bufe A, Wolfertz J, Scheffold T, Lankisch M (2009) Newly detected glucose disturbance is associated with a high prevalence of diastolic dysfunction: double risk for the development of heart failure? Acta Diabetol 46:335–338
Shimabukuro M, Higa N, Asahi T, Yamakawa K, Oshiro Y, Higa M, Masuzaki H (2011) Impaired glucose tolerance, but not impaired fasting glucose, underlies left ventricular diastolic dysfunction. Diabetes Care 34:686–690
Di Cori A, Di Bello V, Miccoli R, Talini E, Palagi C, Delle Donne MG, Penno G, Nardi C, Bianchi C, Mariani M, Del Prato S, Balbarini A (2007) Left ventricular function in normotensive young adults with well-controlled type 1 diabetes mellitus. Am J Cardiol 99:84–90
Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, Seward JB, Tajik AJ (2005) Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr 18:63–68
Ernande L, Rietzschel ER, Bergerot C, De Buyzere ML, Schnell F, Groisne L, Ovize M, Croisille P, Moulin P, Gillebert TC, Derumeaux G (2010) Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: a speckle-tracking imaging study. J Am Soc Echocardiogr 23:1266–1272
Acar G, Akcay A, Sokmen A, Ozkaya M, Guler E, Sokmen G, Kaya H, Nacar AB, Tuncer C (2009) Assessment of atrial electromechanical delay, diastolic functions, and left atrial mechanical functions in patients with type 1 diabetes mellitus. J Am Soc Echocardiogr 22:732–738
Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D (2001) Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol 37:1042–1048
Eren M, Gorgulu S, Uslu N, Celik S, Dagdeviren B, Tezel T (2004) Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart 90:37–43
Mitchell GF, Moyé LA, Braunwald E, Rouleau J-L, Bernstein V, Geltman EM, Flaker GC, Pfeffer MA (1997) Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. Circulation 96:4254–4260
Doering CW, Jalil JE, Janicki JS, Pick R, Aghili S, Abrahams C, Weber K (1988) Collagen network remodelling and diastolic stiffness of the rat left ventricle with pressure overload hypertrophy. Cardiovasc Res 22:686–695
Taegtmeyer H, McNulty P, Young ME (2002) Adaptation and maladaptation of the heart in diabetes: part I General concepts. Circulation 105:1727–1733
Mahgoub MA, Abd-Elfattah AS (1998) Diabetes mellitus and cardiac function. Mol Cell Biochem 180:59–64
Van den Bergh A, Flameng W, Herijgers P (2006) Type II diabetic mice exhibit contractile dysfunction but maintain cardiac output by favourable loading conditions. Eur J Heart Fail 8:777–783
Radovits T, Korkmaz S, Loganathan S, Barnucz E, Bomicke T, Arif R, Karck M, Szabo G (2009) Comparative investigation of the left ventricular pressure–volume relationship in rat models of type 1 and type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol 297:H125–H133
Boudina S, Abel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115:3213–3223
Fischer VW, Barner HB, LaRose LS (1982) Quadriceps and myocardial capillary basal laminae. Their comparison in diabetic patients. Arch Pathol Lab Med 106:336–341
Fischer VW, Barner HB, Larose LS (1984) Pathomorphologic aspects of muscular tissue in diabetes mellitus. Hum Pathol 15:1127–1136
Sutherland CG, Fisher BM, Frier BM, Dargie HJ, More IA, Lindop GB (1989) Endomyocardial biopsy pathology in insulin-dependent diabetic patients with abnormal ventricular function. Histopathology 14:593–602
Kawaguchi M, Techigawara M, Ishihata T, Asakura T, Saito F, Maehara K, Maruyama Y (1997) A comparison of ultrastructural changes on endomyocardial biopsy specimens obtained from patients with diabetes mellitus with and without hypertension. Heart Vessels 12:267–274
Thompson EW (1988) Structural manifestations of diabetic cardiomyopathy in the rat and its reversal by insulin treatment. Am J Anat 182:270–282
Brownlee M, Cerami A, Vlassara H (1988) Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 318:1315–1321
Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM (2003) A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res 92:785–792
Norton GR, Candy G, Woodiwiss AJ (1996) Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation 93:1905–1912
Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H (2004) Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 18:1692–1700
Rohr S (2004) Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res 62:309–322
Berridge MJ (1997) Elementary and global aspects of calcium signalling. J Exp Biol 200:315–319
Bers DM (2002) Cardiac excitation–contraction coupling. Nature 415:198–205
Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Lederer WJ, Matlib MA (2002) Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol 283:H1398–H1408
op den Buijs J, Miklos Z, van Riel NA, Prestia CM, Szenczi O, Toth A, Van der Vusse GJ, Szabo C, Ligeti L, Ivanics T (2005) beta-Adrenergic activation reveals impaired cardiac calcium handling at early stage of diabetes. Life Sci 76:1083–1098
Ren J, Davidoff AJ (1997) Diabetes rapidly induces contractile dysfunctions in isolated ventricular myocytes. Am J Physiol 272:H148–H158
Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS (1983) Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244:E528–E535
Penpargkul S, Fein F, Sonnenblick EH, Scheuer J (1981) Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol 13:303–309
Zhao XY, Hu SJ, Li J, Mou Y, Chen BP, Xia Q (2006) Decreased cardiac sarcoplasmic reticulum Ca2+-ATPase activity contributes to cardiac dysfunction in streptozotocin-induced diabetic rats. J Physiol Biochem 62:1–8
Teshima Y, Takahashi N, Saikawa T, Hara M, Yasunaga S, Hidaka S, Sakata T (2000) Diminished expression of sarcoplasmic reticulum Ca(2+)-ATPase and ryanodine sensitive Ca(2+)Channel mRNA in streptozotocin-induced diabetic rat heart. J Mol Cell Cardiol 32:655–664
Zhong Y, Ahmed S, Grupp IL, Matlib MA (2001) Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am J Physiol Heart Circ Physiol 281:H1137–H1147
Bidasee KR, Dincer UD, Besch HR Jr (2001) Ryanodine receptor dysfunction in hearts of streptozotocin-induced diabetic rats. Mol Pharmacol 60:1356–1364
Trost SU, Belke DD, Bluhm WF, Meyer M, Swanson E, Dillmann WH (2002) Overexpression of the sarcoplasmic reticulum Ca(2+)-ATPase improves myocardial contractility in diabetic cardiomyopathy. Diabetes 51:1166–1171
Belke DD, Swanson EA, Dillmann WH (2004) Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes 53:3201–3208
Yaras N, Ugur M, Ozdemir S, Gurdal H, Purali N, Lacampagne A, Vassort G, Turan B (2005) Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes 54:3082–3088
Vita JA, Keaney JF Jr (2002) Endothelial function: a barometer for cardiovascular risk? Circulation 106:640–642
Drexler H (1998) Factors involved in the maintenance of endothelial function. Am J Cardiol 82:3S–4S
Meigs JB, Hu FB, Rifai N, Manson JE (2004) Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 291:1978–1986
Schalkwijk C, Stehouwer C (2005) Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci 109:143–159
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Forstermann U, Munzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714
Li H, Förstermann U (2000) Nitric oxide in the pathogenesis of vascular disease. J Pathol 190:244–254
Chaudhuri G, Cuevas J, Buga GM, Ignarro LJ (1993) NO is more important than PGI2 in maintaining low vascular tone in feto-placental vessels. Am J Physiol Heart Circ Physiol 265:H2036–H2043
Hamed S, Brenner B, Aharon A, Daoud D, Roguin A (2009) Nitric oxide and superoxide dismutase modulate endothelial progenitor cell function in type 2 diabetes mellitus. Cardiovasc Diabetol 8:56
Festa A, D’Agostino R Jr, Tracy RP, Haffner SM (2002) Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 51:1131–1137
Hirata K, Kuroda R, Sakoda T, Katayama M, Inoue N, Suematsu M, Kawashima S, Yokoyama M (1995) Inhibition of endothelial nitric oxide synthase activity by protein kinase C. Hypertension 25:180–185
Frank PG, Lisanti MP (2008) ICAM-1: role in inflammation and in the regulation of vascular permeability. Am J Physiol Heart Circ Physiol 295:H926–H927
Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P (1986) Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 315:1046–1051
Adameova A, Dhalla NS (2013) Role of microangiopathy in diabetic cardiomyopathy. Heart Fail Rev. doi:10.1007/s10741-013-9378-7
Mortuza R, Chakrabarti S (2013) Glucose-induced cell signaling in the pathogenesis of diabetic cardiomyopathy. Heart Fail Rev. doi:10.1007/s10741-013-9381-z
Sheikh AQ, Hurley JR, Huang W, Taghian T, Kogan A, Cho H, Wang Y, Narmoneva DA (2012) Diabetes alters intracellular calcium transients in cardiac endothelial cells. PLoS ONE 7:e36840
Cheng Y, Guo S, Liu G, Feng Y, Yan B, Yu J, Feng K, Li Z (2012) Transplantation of bone marrow-derived endothelial progenitor cells attenuates myocardial interstitial fibrosis and cardiac dysfunction in streptozotocin-induced diabetic rats. Int J Mol Med 30:870–876
Duvall WL (2005) Endothelial dysfunction and antioxidants. Mt Sinai J Med 72:71–80
Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, Bosi E, Piatti P (2013) l-Arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism 62:255–264
Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN (2000) Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 102:2353–2358
Kawano H, Yasue H, Kitagawa A, Hirai N, Yoshida T, Soejima H, Miyamoto S, Nakano M, Ogawa H (2003) Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab 88:3190–3195
Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim J-a, Quon MJ, Montagnani M (2007) EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 292:E1378–E1387
Hirata Y, Nagata D, Suzuki E, Nishimatsu H, Suzuki J, Nagai R (2010) Diagnosis and treatment of endothelial dysfunction in cardiovascular disease. Int Heart J 51:1–6
Kulkarni AC, Kuppusamy P, Parinandi N (2007) Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid Redox Signal 9:1717–1730
Freinbichler W, Colivicchi MA, Stefanini C, Bianchi L, Ballini C, Misini B, Weinberger P, Linert W, Vareslija D, Tipton KF, Della Corte L (2011) Highly reactive oxygen species: detection, formation, and possible functions. Cell Mol Life Sci 68:2067–2079
Neri M, Fineschi V, Di Paolo M, Pomara C, Riezzo I, Turillazzi E, Cerretani D (2013) Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol. PMID: 23628007
Sultana R, Perluigi M, Allan Butterfield D (2013) Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med 62:157–169
Xu Y, Gu Y, Qian SY (2012) An advanced electron spin resonance (ESR) spin-trapping and LC/(ESR)/MS technique for the study of lipid peroxidation. Int J Mol Sci 13:14648–14666
Bocci V, Valacchi G (2013) Free radicals and antioxidants: how to reestablish redox homeostasis in chronic diseases? Curr Med Chem
Guerra-Araiza C, Alvarez-Mejia AL, Sanchez-Torres S, Farfan-Garcia E, Mondragon-Lozano R, Pinto-Almazan R, Salgado-Ceballos H (2013) Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic Res 47:451–462
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson disease. Free Radic Biol Med 62:13–25
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91 Spec No:179–194
Giergiel M, Lopucki M, Stachowicz N, Kankofer M (2012) The influence of age and gender on antioxidant enzyme activities in humans and laboratory animals. Aging Clin Exp Res 24:561–569
Wang J, Song Y, Wang Q, Kralik PM, Epstein PN (2006) Causes and characteristics of diabetic cardiomyopathy. Rev Diabet Stud 3:108–117
Falcao-Pires I, Leite-Moreira AF (2012) Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev 17:325–344
Khavandi K, Khavandi A, Asghar O, Greenstein A, Withers S, Heagerty AM, Malik RA (2009) Diabetic cardiomyopathy—a distinct disease? Best Pract Res Clin Endocrinol Metab 23:347–360
Ungvari Z, Gupte SA, Recchia FA, Batkai S, Pacher P (2005) Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol 3:221–229
Haidara MA, Yassin HZ, Rateb M, Ammar H, Zorkani MA (2006) Role of oxidative stress in development of cardiovascular complications in diabetes mellitus. Curr Vasc Pharmacol 4:215–227
Selvaraju V, Joshi M, Suresh S, Sanchez JA, Maulik N, Maulik G (2012) Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease—an overview. Toxicol Mech Methods 22:330–335
Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK (2010) Oxidative stress: a key contributor to diabetic cardiomyopathy. Can J Physiol Pharmacol 88:233–240
Ansley DM, Wang B (2013) Oxidative stress and myocardial injury in the diabetic heart. J Pathol 229:232–241
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070
Mellor KM, Ritchie RH, Delbridge LM (2010) Reactive oxygen species and insulin-resistant cardiomyopathy. Clin Exp Pharmacol Physiol 37:222–228
Dirkx E, Schwenk RW, Glatz JF, Luiken JJ, van Eys GJ (2011) High fat diet induced diabetic cardiomyopathy. Prostaglandins Leukot Essent Fatty Acids 85:219–225
Cai L, Kang YJ (2001) Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol 1:181–193
Ishikawa K, Kimura S, Kobayashi A, Sato T, Matsumoto H, Ujiie Y, Nakazato K, Mitsugi M, Maruyama Y (2005) Increased reactive oxygen species and anti-oxidative response in mitochondrial cardiomyopathy. Circ J 69:617–620
Wold LE, Ceylan-Isik AF, Ren J (2005) Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin 26:908–917
Shen X, Zheng S, Metreveli NS, Epstein PN (2006) Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes 55:798–805
Akhileshwar V, Patel SP, Katyare SS (2007) Diabetic cardiomyopathy and reactive oxygen species (ROS) related parameters in male and female rats: a comparative study. Indian J Clin Biochem 22:84–90
Almdal T, Scharling H, Jensen JS, Vestergaard H (2004) The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med 164:1422–1426
Barrett-Connor E, Giardina EG, Gitt AK, Gudat U, Steinberg HO, Tschoepe D (2004) Women and heart disease: the role of diabetes and hyperglycemia. Arch Intern Med 164:934–942
Juutilainen A, Kortelainen S, Lehto S, Ronnemaa T, Pyorala K, Laakso M (2004) Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 27:2898–2904
Bruno G, Giunti S, Bargero G, Ferrero S, Pagano G, Perin PC (2004) Sex-differences in prevalence of electrocardiographic left ventricular hypertrophy in Type 2 diabetes: the Casale Monferrato Study. Diabet Med 21:823–828
Turdi S, Li Q, Lopez FL, Ren J (2007) Catalase alleviates cardiomyocyte dysfunction in diabetes: role of Akt, Forkhead transcriptional factor and silent information regulator 2. Life Sci 81:895–905
Montezano AC, Touyz RM (2012) Reactive oxygen species and endothelial function—role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol 110:87–94
Maalouf RM, Eid AA, Gorin YC, Block K, Escobar GP, Bailey S, Abboud HE (2012) Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol 302:C597–C604
Octavia Y, Brunner-La Rocca HP, Moens AL (2012) NADPH oxidase-dependent oxidative stress in the failing heart: from pathogenic roles to therapeutic approach. Free Radic Biol Med 52:291–297
Cho HY, Reddy SP, Kleeberger SR (2006) Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 8:76–87
Li B, Liu S, Miao L, Cai L (2012) Prevention of diabetic complications by activation of Nrf2: diabetic cardiomyopathy and nephropathy. Exp Diabetes Res 2012:216512
Bhagavan HN, Chopra RK (2007) Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 7(Suppl):S78–S88
Littarru GP, Tiano L, Belardinelli R, Watts GF (2011) Coenzyme Q(10), endothelial function, and cardiovascular disease. BioFactors 37:366–373
Huynh K, Kiriazis H, Du XJ, Love JE, Gray SP, Jandeleit-Dahm KA, McMullen JR, Ritchie RH (2013) Targeting the upregulation of reactive oxygen species subsequent to hyperglycemia prevents type 1 diabetic cardiomyopathy in mice. Free Radic Biol Med 60:307–317
Turan B (2010) Role of antioxidants in redox regulation of diabetic cardiovascular complications. Curr Pharm Biotechnol 11:819–836
Apostolova N, Blas-Garcia A, Esplugues JV (2011) Mitochondria sentencing about cellular life and death: a matter of oxidative stress. Curr Pharm Des 17:4047–4060
Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P (2010) Oxidative stress: emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimers Dis 20(Suppl 2):S453–S473
Holmuhamedov EL, Jovanovic S, Dzeja PP, Jovanovic A, Terzic A (1998) Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am J Physiol 275:H1567–H1576
Kowaltowski AJ (2000) Alternative mitochondrial functions in cell physiopathology: beyond ATP production. Braz J Med Biol Res 33:241–250
Mammucari C, Rizzuto R (2010) Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev 131:536–543
Scherz-Shouval R, Elazar Z (2007) ROS, mitochondria and the regulation of autophagy. Trends Cell Biol 17:422–427
Sordahl LA (1979) Role of mitochondria in heart cell function. Tex Rep Biol Med 39:5–18
Williamson CL, Dabkowski ER, Baseler WA, Croston TL, Alway SE, Hollander JM (2010) Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am J Physiol Heart Circ Physiol 298:H633–H642
Di Lisa F, Canton M, Menabo R, Kaludercic N, Bernardi P (2007) Mitochondria and cardioprotection. Heart Fail Rev 12:249–260
Tanaka Y, Konno N, Kako KJ (1992) Mitochondrial dysfunction observed in situ in cardiomyocytes of rats in experimental diabetes. Cardiovasc Res 26:409–414
Momiyama Y, Atsumi Y, Ohsuzu F, Ui S, Morinaga S, Matsuoka K, Kimura M (1999) Rapid progression of cardiomyopathy in mitochondrial diabetes. Jpn Circ J 63:130–132
Sack MN (2009) Type 2 diabetes, mitochondrial biology and the heart. J Mol Cell Cardiol 46:842–849
Dobrin JS, Lebeche D (2010) Diabetic cardiomyopathy: signaling defects and therapeutic approaches. Expert Rev Cardiovasc Ther 8:373–391
Bugger H, Abel ED (2010) Mitochondria in the diabetic heart. Cardiovasc Res 88:229–240
Duncan JG (2011) Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta 1813:1351–1359
Adeghate E, Singh J (2013) Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail Rev. doi:10.1007/s10741-013-9388-5
Feuvray D, Darmellah A (2008) Diabetes-related metabolic perturbations in cardiac myocyte. Diabetes Metab 34(Suppl 1):S3–S9
Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD (2009) Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol 54:1891–1898
Lin G, Brownsey RW, MacLeod KM (2009) Regulation of mitochondrial aconitase by phosphorylation in diabetic rat heart. Cell Mol Life Sci 66:919–932
Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED (2009) Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 119:1272–1283
Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J (2010) NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res 85:473–483
Nakamura H, Matoba S, Iwai-Kanai E, Kimata M, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M, Mita Y, Ikeda K, Okigaki M, Adachi S, Tanaka H, Takamatsu T, Matsubara H (2012) p53 promotes cardiac dysfunction in diabetic mellitus caused by excessive mitochondrial respiration-mediated reactive oxygen species generation and lipid accumulation. Circ Heart Fail 5:106–115
Murphy E, Wong R, Steenbergen C (2008) Signalosomes: delivering cardioprotective signals from GPCRs to mitochondria. Am J Physiol Heart Circ Physiol 295:H920–H922
Latronico MV, Condorelli G (2012) The might of microRNA in mitochondria. Circ Res 110:1540–1542
Dabkowski ER, Williamson CL, Bukowski VC, Chapman RS, Leonard SS, Peer CJ, Callery PS, Hollander JM (2009) Diabetic cardiomyopathy-associated dysfunction in spatially distinct mitochondrial subpopulations. Am J Physiol Heart Circ Physiol 296:H359–H369
Fancher IS, Dick GM, Hollander JM (2013) Diabetes mellitus reduces the function and expression of ATP-dependent K(+) channels in cardiac mitochondria. Life Sci 92:664–668
Gucek M, Murphy E (2010) What can we learn about cardioprotection from the cardiac mitochondrial proteome? Cardiovasc Res 88:211–218
Murphy E, Steenbergen C (2011) What makes the mitochondria a killer? Can we condition them to be less destructive? Biochim Biophys Acta 1813:1302–1308
Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, Hollander JM (2011) Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol 300:R186–R200
Essop MF, Chan WA, Hattingh S (2011) Proteomic analysis of mitochondrial proteins in a mouse model of type 2 diabetes. Cardiovasc J Afr 22:175–178
Bolisetty S, Jaimes EA (2013) Mitochondria and reactive oxygen species: physiology and pathophysiology. Int J Mol Sci 14:6306–6344
Gao L, Laude K, Cai H (2008) Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet Clin North Am Small Anim Pract 38:137–155, vi
Paradies G, Petrosillo G, Paradies V, Ruggiero FM (2009) Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium 45:643–650
Parinandi NL, Weis BK, Schmid HH (1988) Assay of cardiolipin peroxidation by high-performance liquid chromatography. Chem Phys Lipids 49:215–220
Shi Y (2010) Emerging roles of cardiolipin remodeling in mitochondrial dysfunction associated with diabetes, obesity, and cardiovascular diseases. J Biomed Res 24:6–15
Wong R, Steenbergen C, Murphy E (2012) Mitochondrial permeability transition pore and calcium handling. Methods Mol Biol 810:235–242
Han X, Yang J, Yang K, Zhao Z, Abendschein DR, Gross RW (2007) Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry 46:6417–6428
Han X, Yang J, Cheng H, Yang K, Abendschein DR, Gross RW (2005) Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry 44:16684–16694
Saini-Chohan HK, Holmes MG, Chicco AJ, Taylor WA, Moore RL, McCune SA, Hickson-Bick DL, Hatch GM, Sparagna GC (2009) Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J Lipid Res 50:1600–1608
Kiebish MA, Yang K, Sims HF, Jenkins CM, Liu X, Mancuso DJ, Zhao Z, Guan S, Abendschein DR, Han X, Gross RW (2012) Myocardial regulation of lipidomic flux by cardiolipin synthase: setting the beat for bioenergetic efficiency. J Biol Chem 287:25086–25097
Goldstein A, Wolfe LA (2013) The elusive magic pill: finding effective therapies for mitochondrial disorders. Neurotherapeutics 10:320–328
Acknowledgments
This study was supported by National Institute of Health Grants HL-56803 and HL-69910 to NM. We would like to acknowledge Shereen Cynthia D’Cruz, Ph.D and Joshua Goldman for their assistance during the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joshi, M., Kotha, S.R., Malireddy, S. et al. Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Mol Cell Biochem 386, 233–249 (2014). https://doi.org/10.1007/s11010-013-1861-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-013-1861-x