Abstract
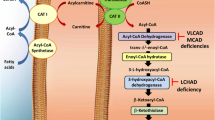
The accumulation of octanoic (OA) and decanoic (DA) acids in tissue is the common finding in medium-chain acyl-coenzyme A dehydrogenase deficiency (MCADD), the most frequent defect of fatty acid oxidation. Affected patients present hypoketotic hypoglycemia, rhabdomyolysis, hepatomegaly, seizures and lethargy, which may progress to coma and death. At present, the pathophysiological mechanisms underlying hepatic and skeletal muscle alterations in affected patients are poorly known. Therefore, in the present work, we investigated the in vitro effects of OA and DA, the accumulating metabolites in MCADD, on various bioenergetics and oxidative stress parameters. It was verified that OA and DA decreased complexes I–III, II–III and IV activities in liver and also inhibit complex IV activity in skeletal muscle. In addition, DA decreased complexes II–III activity in skeletal muscle. We also verified that OA and DA increased TBA-RS levels and carbonyl content in both tissues. Finally, DA, but not OA, significantly decreased GSH levels in rat skeletal muscle. Our present data show that the medium-chain fatty acids that accumulate in MCADD impair electron transfer through respiratory chain and elicit oxidative damage in rat liver and skeletal muscle. It may be therefore presumed that these mechanisms are involved in the pathophysiology of the hepatopathy and rhabdomyolysis presented by MCADD-affected patients.



Similar content being viewed by others
References
Rinaldo P, Matern D, Bennett MJ (2002) Fatty acid oxidation disorders. Annu Rev Physiol 64:477–502. doi:10.1146/annurev.physiol.64.082201.154705
Lindner M, Hoffmann GF, Matern D (2010) Newborn screening for disorders of fatty-acid oxidation: experience and recommendations from an expert meeting. J Inherit Metab Dis 33:521–526. doi:10.1007/s10545-010-9076-8
Onkenhout W, Venizelos V, Van der Poel PFH, Van der Heuvel MPM, Poorthuis BJHM (1995) Identification and quantification of intermediates of unsaturated fatty acid metabolism in plasma of patients with fatty acid oxidation disorders. Clin Chem 41:1467–1474
Ruitenbeek W, Poels PJ, Turnbull DM, Garavaglia B, Chalmers RA, Taylor RW, Gabreels FJ (1995) Rhabdomyolysis and acute encephalopathy in late onset medium chain acyl-CoA dehydrogenase deficiency. J Neurol Neurosurg Psychiatry 58:209–214. doi:10.1136/jnnp.58.2.209
Raymond K, Bale AE, Barnes CA, Rinaldo P (1999) Medium-chain acyl Co-A dehydrogenase deficiency: sudden and unexpected death of a 45 year old woman. Genet Med 1:293–294
Feillet F, Steinmann G, Vianey-Saban C, Chillou C, Sadoul N, Lefebvre E, Vidailhet M, Bollaert PE (2003) Adult presentation of MCAD deficiency revealed by coma and severe arrhythmias. Intensive Care Med 29:1594–1597. doi:10.1007/s00134-003-1871-3
Roe CR, Ding JH (2001) Mitochondrial fatty acid oxidation disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1909–1963
Rinaldo P, Hahn S, Matern D (2006) Inborn errors of amino acid, organic acid, and fatty acid metabolism. In: Burtis CA, Ashwood ER, Bruns DE (eds) Tietz textbook of clinical chemistry and molecular diagnostics, 4th edn. Elsevier, St. Louis, pp 2207–2247
Assis DR, Ribeiro CA, Rosa RB, Schuck PF, Dalcin KB, Vargas CR, Wannmacher CM, Dutra-Filho CS, Wyse AT, Briones P, Wajner M (2003) Evidence that antioxidants prevent the inhibition of Na+ , K+ -ATPase activity induced by octanoic acid in rat cerebral cortex in vitro. Neurochem Res 28:1255–1263. doi:10.1023/A:1024244915832
Assis DR, Maria RC, Rosa RB, Schuck PF, Ribeiro CA, Ferreira GC, Dutra-Filho CS, Wyse AT, Wannmacher CM, Perry ML, Wajner M (2004) Inhibition of energy metabolism in cerebral cortex of young rats by the medium-chain fatty acids accumulating in MCAD deficiency. Brain Res 1030:141–151. doi:10.1016/j.brainres.2004.10.010
Schuck PF, Ferreira GC, Moura AP, Busanello EN, Tonin AM, Dutra-Filho CS, Wajner M (2009) Medium-chain fatty acids accumulating in MCAD deficiency elicit lipid and protein oxidative damage and decrease non-enzymatic antioxidant defenses in rat brain. Neurochem Int 54:519–525. doi:10.1016/j.neuint.2009.02.009
Schuck PF, Ferreira GC, Tonin AM, Viegas CM, Busanello EN, Moura AP, Zanatta A, Klamt F, Wajner M (2009) Evidence that the major metabolites accumulating in medium-chain acyl-CoA dehydrogenase deficiency disturb mitochondrial energy homeostasis in rat brain. Brain Res 1296:117–126. doi:10.1016/j.brainres.2009.08.053
Evelson P, Travacio M, Repetto M, Escobar J, Llesuy S, Lissi EA (2001) Evaluation of total reactive antioxidant potential (TRAP) of tissue homogenates and their cytosols. Arch Biochem Biophys 388:261–266. doi:10.1006/abbi.2001.2292
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders M, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36. doi:10.1016/0009-8981(85)90135-4
Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55:2142–2145. doi:10.1111/j.1471-4159.1990.tb05809.x
Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM (1994) A biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51. doi:10.1016/0009-8981(94)90055-8
Silva CG, Ribeiro CA, Leipnitz G, Dutra-Filho CS, Wyse AT, Wannmacher CM, Sarkis JJ, Jakobs C, Wajner M (2002) Inhibition of cytochrome c oxidase activity in rat cerebral cortex and human skeletal muscle by D-2-hydroxyglutaric acid in vitro. Biochim Biophys Acta 1586:81–91. doi:10.1016/S0925-4439(01)00088-6
Schuck PF, Leipnitz G, Ribeiro CA, Dalcin KB, Assis DR, Barschak AG, Pulrolnik V, Wannmacher CM, Wyse AT, Wajner M (2002) Inhibition of creatine kinase activity in vitro by ethylmalonic acid in cerebral cortex of young rats. Neurochem Res 27:1633–1639. doi:10.1023/A:1021682910373
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. doi:10.1016/0076-6879(90)86134-H
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363. doi:10.1016/S0076-6879(94)33041-7
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352. doi:10.1385/0-89603-472-0:347
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Perper JA, Ahdab-Barmada M (1992) Fatty liver, encephalopathy, and sudden unexpected death in early childhood due to medium-chain acyl-coenzyme A dehydrogenase deficiency. Am J Forensic Med Pathol 13:329–334. doi:10.1097/00000433-199212000-00013
Davis FB, Davis PJ, Blas SD, Schoeln M (1987) Action of long-chain fatty acids in vitro on Ca2+ -stimulatable, Mg2+ -dependent ATPase activity in human red cell membranes. Biochem J 248:511–516
Rinaldo P (2001) Fatty acid transport and mitochondrial oxidation disorders. Semin Liver Dis 21:489–500. doi:10.1055/s-2001-19037
Scholz R, Schwabe U, Soboll S (1984) Influence of fatty acids on energy metabolism. 1. Stimulation of oxygen consumption, ketogenesis and CO2 production following addition of octanoate and oleate in perfused rat liver. Eur J Biochem 141:223–230. doi:10.1111/j.1432-1033.1984.tb08179.x
Soboll S, Gründel S, Schwabe U, Scholz R (1984) Influence of fatty acids on energy metabolism. 2. Kinetics of changes in metabolic rates and changes in subcellular adenine nucleotide contents and pH gradients following addition of octanoate and oleate in perfused rat liver. Eur J Biochem 141:231–236. doi:10.1111/j.1432-1033.1984.tb08180.x
Morand C, Besson C, Demigne C, Remesy C (1994) Importance of the modulation of glycolysis in the control of lactate metabolism by fatty acids in isolated hepatocytes from fed rats. Arch Biochem Biophys 309:254–260. doi:10.1006/abbi.1994.1110
Clay VJ, Ragan CI (1988) Evidence for the existence of tissue specific isoenzymes of mitochondrial NADH dehydrogenase. Biochem Biophys Res Commun 157:1423–1428. doi:10.1016/S0006-291X(88)81034-9
Merle P, Kadenbach B (1980) On the heterogeneity of vertebrate cytochrome c oxidase polypeptide chain composition. Hoppe Seylers Z Physiol Chem 361:1257–1259. doi:10.1515/bchm2.1980.361.2.1257
Taylor RW, Birch-Machin MA, Bartlett K, Turnbull DM (1993) Succinate-cytochrome c reductase: assessment of its value in the investigation of defects of the respiratory chain. Biochim Biophys Acta 1181:261–265. doi:10.1016/0925-4439(93)90030-5
Pettenuzzo LF, Ferreira GC, Schmidt AL, Dutra-Filho CS, Wyse AT, Wajner M (2006) Differential inhibitory effects of methylmalonic acid on respiratory chain complex activities in rat tissues. Int J Dev Neurosci 24:45–52. doi:10.1016/j.ijdevneu.2005.10.005
Halliwell B, Gutteridge JMC (1999) Detection of free radicals and others reactive species: trapping and fingerprinting. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, Oxford, pp 351–425
Lissi E, Salim-Hanna M, Pascual C, Castillo MD (1995) Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Rad Biol Med 18:153–158. doi:10.1016/0891-5849(94)00117-3
Duran M, Wadman SK (1987) Chemical diagnosis of inherited defects of fatty acid metabolism and ketogenesis. Enzyme 38:115–123
Martínez G, Jiménez-Sánchez G, Divry P, Vianey-Saban C, Riudor E, Rodés M, Briones P, Ribes A (1997) Plasma free fatty acids in mitochondrial fatty acid oxidation defects. Clin Chim Acta 267:143–154. doi:10.1016/S0009-8981(97)00130-7
Acknowledgments
This work was supported by grants from CNPq, FAPESC and PIBIC/UNESC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scaini, G., Simon, K.R., Tonin, A.M. et al. Toxicity of octanoate and decanoate in rat peripheral tissues: evidence of bioenergetic dysfunction and oxidative damage induction in liver and skeletal muscle. Mol Cell Biochem 361, 329–335 (2012). https://doi.org/10.1007/s11010-011-1119-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-1119-4