Abstract
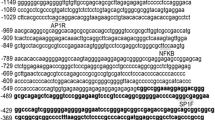
TIGAR expression resulted in down-regulation of glycolysis, reduction of intracellular levels of reactive oxygen species, and protection from apoptosis. Despite biological importance, its promoter has not yet been characterized. In this study, we characterized that transcription factor SP1 plays a pivotal role for basal activity of TIGAR promoter. By 5′RACE, the transcription start site was identified locating at 134 bp upstream of the translation initiation site. Different portions of 5′-flanking and 5′-untranslated regions were fused to a luciferase reporter gene to create reporter plasmids, and constructs were transiently transfected into HepG2, Bel-7402, and Smmc-7721 cell lines for luciferase analysis. A minimal region −56/−4 bearing a SP1-binding site was characterized and plays a vital role. Data from electrophoretic mobility shift assay and chromatin immunoprecipitation showed that SP1 can interact with the SP1-binding site within TIGAR promoter in vitro and in vivo. Conclusively, SPl is indispensable for basal activity of TIGAR promoter.




Similar content being viewed by others
Abbreviations
- RACE:
-
Rapid amplification of cDNA ends
- TSS:
-
Transcriptional start site
- EMSA:
-
Electrophoretic mobility shift assays
- ChIP:
-
Chromatin immunoprecipitation
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics. CA Cancer J Clin 55(2002):74–108
Bensaad K, Tsuruta A, Selak MA et al (2006) TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126:107–120
Bartrons R, Caro J (2007) Hypoxia, glucose metabolism and the Warburg’s effect. J Bioenerg Biomembr 39:223–229
Gao G, Sebastian B, Jochen H et al (2008) A short ultra conserved sequence drives transcription from an alternate FBN1 promoter. Int J Biochem Cell Biol 40:638–650
Bensaad K, Cheung ER, Vousden KH (2009) Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J 28:3015–3026
Kadonaga JT, Carner KR, Masiarz FR et al (1987) Isolation of cDNA encoding transcription factor SP1 and functional analysis of the DNA binding domain. Cell 51:1079–1090
Wierstra I (2008) SP1: emerging roles—beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun 372:1–7
Smale ST, Schmidt MC, Berk AJ (1990) Transcriptional activation by SP1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci USA 87:4509–4513
Emili A, Greenblat J, Ingles CJ (1994) Species-specific interaction of the glutamine-rich activation domain of SP1 with the TATA box-binding protein. Mol Cell Biol 14:1582–1593
Tanese N, Saluja D, Vassallo MF et al (1996) Molecular cloning and analysis of two subunits of the human TFIID complex: hTAFII130 and hTAFII100. Proc Natl Acad Sci USA 93:13611–13616
Saffer JD, Jackson SP, Annarella MB (1991) Developmental expression of SP1 in the mouse. Mol Cell Biol 11:2189–2199
Black AR, Jensen D, Lin SY et al (1999) Growth/cell cycle regulation of SP1 phosphorylation. J Biol Chem 274:1207–1215
Kavurma MM, Santiago FS, Bonfoco E et al (2001) SP1 phosphorylation regulates apoptosis via extracellular FasL-Fas engagement. J Biol Chem 276:4964–4971
Milavetz BI (2002) SP1 and AP-1 elements direct chromatin remodeling in SV40 chromosomes during the first 6 hours of infection. Virology 294:170–179
Wang JY, Liu XF, Ni PH et al (2010) SP1 is required for basal activation and chromatin accessibility of CD151 promoter in liver cancer cells. Biochem Biophys Res Commun 393:291–296
Koutsodontis G, Tentes I, Papakosta P et al (2001) Sp1 plays a critical role in the transcriptional activation of the human cyclin-dependent kinase inhibitor p21 (WAF1/Cip1) gene by the p53 tumor suppressor protein. J Biol Chem 276:29116–29125
Koutsodontis G, Vasilaki E, Chou WC et al (2005) Physical and functional interactions between members of the tumor suppressor p53 and the Sp families of transcription factors: importance for the regulation of genes involved in cell-cycle arrest and apoptosis. Biochem J 389:443–455
Acknowledgment
We are grateful to Prof Jingyi Hui (Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for providing research facilities and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, S., Gu, Z., Ni, P. et al. SP1 plays a pivotal role for basal activity of TIGAR promoter in liver cancer cell lines. Mol Cell Biochem 359, 17–23 (2012). https://doi.org/10.1007/s11010-011-0993-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0993-0