Abstract
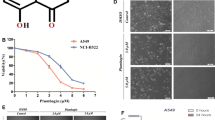
Caveolin-1 (Cav-1) expression frequently found in lung cancer was linked with disease prognosis and progression. This study reveals for the first time that Cav-1 sensitizes cisplatin-induced lung carcinoma cell death by the mechanism involving oxidative stress modulation. We established stable Cav-1 overexpressed (H460/Cav-1) cells and investigated their cisplatin susceptibility in comparison with control-transfected cells and found that Cav-1 expression significantly enhanced cisplatin-mediated cell death. Results indicated that the different response to cisplatin between these cells was resulted from different level of superoxide anion induced by cisplatin. Inhibitory study revealed that superoxide anion inhibitor MnTBAP could inhibit cisplatin-mediated toxicity only in H460/Cav-1 cells while had no effect on H460 cells. Further, superoxide anion detected by DHE probe indicated that H460/Cav-1 cells generated significantly higher superoxide anion level in response to cisplatin than that of control cells. The role of Cav-1 in regulating cisplatin sensitivity was confirmed in shRNA-mediated Cav-1 down-regulated (H460/shCav-1) cells and the cells exhibited decreased cisplatin susceptibility and superoxide generation. In summary, these findings reveal novel aspects regarding role of Cav-1 in modulating oxidative stress induced by cisplatin, possibly providing new insights for cancer biology and cisplatin-based chemotherapy.





Similar content being viewed by others
References
Sleijfer DT, Meijer S, Mulder NH (1985) Cisplatin: a review of clinical applications and renal toxicity. Pharm World Sci 7:237–244
Miyajima A, Nakashima J, Yoshioka K, Tachibana M, Tazaki H, Mural M (1997) Role of reactive oxygen species in cis-dichlorodiammineplatinum-induced cytotoxicity on bladder cancer cells. Br J Cancer 76:206–210
Bragado P, Armesilla A, Silva A, Porras A (2007) Apoptosis by cisplatin requires p53 mediated p38α MAPK activation through ROS generation. Apoptosis 12:1733–1742
Wang L, Chanvorachote P, Toledo D, Stehlik C, Mercer RR, Castranova V, Rojanasakul Y (2008) Peroxide is a key mediator of Bcl-2 down-regulation and apoptosis induction by cisplatin in human lung cancer cells. Mol Pharmacol 73:119–127
Chanvorachote P, Nimmannit U, Stehlik C et al (2006) Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res 66:6353–6360
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82:291–295
Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35:1147–1150
Laurent A, Nicco C, Chéreau C et al (2005) Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res 65:948–956
Storz P (2005) Reactive oxygen species in tumor progression. Front Biosci 10:1881–1896
Teramoto S, Tomita T, Matsui H, Ohga E, Matsuse T, Ouchi Y (1999) Hydrogen peroxide-induced apoptosis and necrosis in human lung fibroblasts: protective roles of glutathione. Jpn J Pharmacol 79:33–40
Jiang M, Wei Q, Pabla N et al (2007) Effects of hydroxyl radical scavenging on cisplatin-induced p53 activation, tubular cell apoptosis and nephrotoxicity. Biochem Pharmacol 73:1499–1510
Ngo C, Chereau C, Nicco C, Weill B, Chapron C, Batteux F (2009) Reactive oxygen species controls endometriosis progression. Am J Pathol 175:225–234
Burgermeister E, Liscovitch M, Röcken C, Schmid RM, Ebert MPA (2008) Caveats of caveolin-1 in cancer progression. Cancer Lett 268:187–201
Couet J, Belanger MM, Roussel E, Drolet MC (2001) Cell biology of caveolae and caveolin. Adv Drug Deliv Rev 49:223–235
Ravid D, Maor S, Werner H, Liscovitch M (2006) Caveolin-1 inhibits anoikis and promotes survival signaling in cancer cells. Adv Enzym Regul 46:163–175
Wu P, Wang X, Li F et al (2008) Growth suppression of MCF-7 cancer cell-derived xenografts in nude mice by caveolin-1. Biochem Biophys Res Commun 376:215–220
Chanvorachote P, Nimmannit U, Lu Y, Talbott S, Jiang B-H, Rojanasakul Y (2009) Nitric oxide regulates lung carcinoma cell anoikis through inhibition of ubiquitin-proteasomal degradation of caveolin-1. J Biol Chem 284:28476–28484
Luanpitpong S, Talbott SJ, Rojanasakul Y et al (2010) Regulation of lung cancer cell migration and invasion by reactive oxygen species and Caveolin-1. J Biol Chem M110:124958. doi:10.1074
Ho C-C, Kuo S-H, Huang P-H, Huang H-Y, Yang C-H, Yang P-C (2008) Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer 59:105–110
Yoo SH, Park YS, Kim HR et al (2003) Expression of caveolin-1 is associated with poor prognosis of patients with squamous cell carcinoma of the lung. Lung cancer 42:195–202
Yang CPH, Galbiati F, Volonté D, Horwitz SB, Lisanti MP (1998) Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett 439:368–372
Bélanger MM, Gaudreau M, Roussel E, Couet J (2004) Role of caveolin-1 in etoposide resistance development in A549 lung cancer cells. Cancer Biol Ther 3:954–959
Park J, Bae E, Lee C et al (2010) RNA interference-directed caveolin-1 knockdown sensitizes SN12CPM6 cells to doxorubicin-induced apoptosis and reduces lung metastasis. Tumor Biol 31:643–650
Tirado OM, MacCarthy CM, Fatima N, Villar J, Mateo-Lozano S, Notario V (2010) Caveolin-1 promotes resistance to chemotherapy-induced apoptosis in Ewing’s sarcoma cells by modulating PKCα phosphorylation. Int J Cancer 126:426–436
Nakatani K, Wada T, Nakamura M, Uzawa K, Tanzawa H, Fujita S (2005) Expression of caveolin-1 and its correlation with cisplatin sensitivity in oral squamous cell carcinoma. J Cancer Res Clin 131:445–452
Shajahan AN, Wang A, Decker M, Minshall RD, Liu MC, Clarke R (2007) Caveolin-1 tyrosine phosphorylation enhances paclitaxel-mediated cytotoxicity. J Biol Chem 282:5934–5943
Kartalou M, Essigmann JM (2001) Mechanisms of resistance to cisplatin. Mutat Res Fundam Mol Mech Mugag 478:23–43
Stewart DJ (2007) Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol 63:12–31
Rungtabnapa P, Nimmannit U, Halim H, Rojanasakul Y, Chanvorachote P (2011) Hydrogen peroxide inhibits non-small cell lung cancer cell anoikis through the inhibition of caveolin-1 degradation. Am J Physiol Cell Physiol 300:C235–C245
Kato K, Hida Y, Miyamoto M (2002) Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Am Cancer Soc 94:929–933
Williams TM, Hassan GS, Li J et al (2005) Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer. J Biol Chem 280:25134–25145
Dasari A, Bartholomew JN, Volonte D, Galbiati F (2006) Oxidative induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res 66:10805–10814
Chretien A, Piront N, Delaive E, Demazy C, Ninane N, Toussaint O (2008) Increased abundance of cytoplasmic and nuclear caveolin 1 in human diploid fibroblasts in H2O2-induced premature senescence and interplay with p38αMAPK. FEBS Lett 582:1685–1692
Linge A, Weinhold K, Blasche R, Kasper M, Barth K (2007) Downregulation of caveolin-1 affects bleomycin-induced growth arrest and cellular senescence in A549 cells. Int J Biochem Cell Biol 39:1964–1974
Bartholomew JN, Dasari A, Galbiati F (2009) Caveolin-1 regulates the antagonistic pleiotropic properties of cellular senescence through a novel Mdm2/p53-mediated pathway. Cancer Res 69:2878–2886
Okamoto T, Schlegel A, Scherer PE, Lisanti MP (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273:5419–5422
Williams TM, Lisanti MP (2005) Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol 28:C494–C506
Sunaga N, Miyajima K, Suzuki M et al (2004) Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res 64:4277–4285
Salganik RI (2001) The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr 20:464S–472S
Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219:1–14
Acknowledgments
We would like to thank Mr. Krich Rajprasit and Mr. Hasseri Halim. This study was supported by Ratchadaphiseksomphot Endowment Fund, Chulalongkorn University (Chanvorachote P.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pongjit, K., Chanvorachote, P. Caveolin-1 sensitizes cisplatin-induced lung cancer cell apoptosis via superoxide anion-dependent mechanism. Mol Cell Biochem 358, 365–373 (2011). https://doi.org/10.1007/s11010-011-0988-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0988-x