Abstract
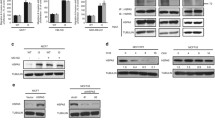
Histone deacetylase 6 (HDAC6) inhibition, recently, has been shown to promote the acetylation of heat-shock protein 90 (Hsp90) and disrupt its chaperone function. Her-2 oncoprotein is identified as a client protein of Hsp90. Therefore, in this study we examined the effect of carbamazepine, which could inhibit HDAC on Hsp90 acetylation and Her-2 stability. The results of this study demonstrate that while carbamazepine had no effect on the Her-2 mRNA level, it induced Her-2 protein degradation via the proteasome pathway by disrupting the chaperone function of Hsp90 in SK-BR-3 cells. Mechanistically, carbamazepine could enhance the acetylation of α-tubulin, indicating its inhibitory effect on HDAC6. Functionally, carbamazepine could synergize with trastuzumab or geldanamycin to promote Her-2 degradation and inhibit breast cancer cell proliferation. Thus, this study has potential clinical implications by providing a promising strategy to overcome the development of resistance against trastuzumab therapy for breast cancer.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17:2639–2648
Andersson J, Linderholm B, Greim G, Lindh B, Lindman H, Tennvall J, Tennvall-Nittby L, Pettersson-Sköld D, Sverrisdottir A, Söderberg M, Klaar S, Bergh J (2002) A population-based study on the first forty-eight breast cancer patients receiving trastuzumab (Herceptin) on a named patient basis in Sweden. Acta Oncol 41:276–281
Engel RH, Kaklamani VG (2007) HER2-positive breast cancer: current and future treatment strategies. Drugs 67:1329–1341
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707–712
Pils D, Pinter A, Reibenwein J, Alfanz A, Horak P, Schmid BC, Hefler L, Horvat R, Reinthaller A, Zeillinger R, Krainer M (2007) In ovarian cancer the prognostic influence of HER2/neu is not dependent on the CXCR4/SDF-1 signalling pathway. Br J Cancer 96:485–491
Shawver LK, Slamon D, Ullrich A (2002) Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell 1:117–123
McKeage K, Perry CM (2002) Trastuzumab: a review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs 62:209–243
Goetz MP, Toft DO, Ames MM, Erlichman C (2003) The Hsp90 chaperone complex as a novel target for cancer therapy. Ann Oncol 14:1169–1176
Hartson SD, Irwin AD, Shao J, Scroggins BT, Volk L, Huang W, Matts RL (2000) p50(cdc37) is a nonexclusive Hsp90 cohort which participates intimately in Hsp90-mediated folding of immature kinase molecules. Biochemistry 39:7631–7644
Young JC, Moarefi I, Hartl FU (2001) Hsp90: a specialized but essential protein-folding tool. J Cell Biol 154:267–273
Terry J, Lubieniecka JM, Kwan W, Liu S, Nielsen TO (2005) Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin prevents synovial sarcoma proliferation via apoptosis in in vitro models. Clin Cancer Res 11:5631–5638
Seigneurin-Berny D, Verdel A, Curtet S, Lemercier C, Garin J, Rousseaux S, Khochbin S (2001) Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol Cell Biol 21:8035–8044
Boyault C, Zhang Y, Fritah S, Caron C, Gilquin B, Kwon SH, Garrido C, Yao TP, Vourc’h C, Matthias P, Khochbin S (2007) HDAC6 controls major cell response pathways to cytotoxic accumulation of protein aggregates. Genes Dev 21:2172–2181
Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. Embo J 22:1168–1179
Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP (2005) HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18:601–607
Beutler AS, Li S, Nicol R, Walsh MJ (2005) Carbamazepine is an inhibitor of histone deacetylases. Life Sci 76:3107–3115
Kuminek G, Kratz JM, Ribeiro R, Kelmann RG, de Araújo BV, Teixeira HF, Simões CM, Koester LS (2009) Pharmacokinetic study of a carbamazepine nanoemulsion in beagle dogs. Int J Pharm 378:146–148
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Yu X, Guo ZX, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS (2002) Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst 94:504–513
Guardiola AR, Yao TP (2002) Molecular cloning and characterization of a novel histone deacetylase HDAC10. J Biol Chem 277:3350–3356
McLaughlin SH, Sobott F, Yao ZP, Zhang W, Nielsen PR, Grossmann JG, Laue ED, Robinson CV, Jackson SE (2006) The co-chaperone p23 arrests the Hsp90 ATPase cycle to trap client proteins. J Mol Bio 356:746–758
Kelly WK, Marks PA (2005) Drug insight: histone deacetylase inhibitors—development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol 2:150–157
Lam PB, Burga LN, Wu BP, Hofstatter EW, Lu KP, Wulf GM (2008) Prolyl isomerase Pin1 is highly expressed in Her2-positive breast cancer and regulates erbB2 protein stability. Mol Cancer 7:91
Xu W, Soga S, Beebe K, Lee MJ, Kim YS, Trepel J, Neckers L (2007) Sensitivity of epidermal growth factor receptor and ErbB2 exon 20 insertion mutants to Hsp90 inhibition. Br J Cancer 97:741–744
Xu W, Neckers L (2007) Targeting the molecular chaperone heat shock protein 90 provides a multifaceted effect on diverse cell signaling pathways of cancer cells. Clin Cancer Res 13:1625–1629
Fiskus W, Ren Y, Mohapatra A, Bali P, Mandawat A, Rao R, Herger B, Yang Y, Atadja P, Wu J, Bhalla K (2007) Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-alpha levels and transcriptional activity: a result of hyperacetylation and inhibition of chaperone function of heat shock protein 90. Clin Cancer Res 13:4882–4890
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K (2005) Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem 280:26729–26734
Blagosklonny MV, Robey R, Sackett DL, Du L, Traganos F, Darzynkiewicz Z, Fojo T, Bates SE (2002) Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol Cancer Ther 1:937–941
Furumai R, Matsuyama A, Kobashi N, Lee KH, Nishiyama M, Nakajima H, Tanaka A, Komatsu Y, Nishino N, Yoshida M, Horinouchi S (2002) FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res 62:4916–4921
Remy S, Beck H (2006) Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain 129:18–35
Acknowledgment
This study was supported by grants from Heilongjiang Provincial Bureau of Health (2009015) and from the Tumor Hospital of Harbin Medical University (JJ2007-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Qingwei Meng, Xuesong Chen, and Lichun Sun have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Meng, Q., Chen, X., Sun, L. et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem 348, 165–171 (2011). https://doi.org/10.1007/s11010-010-0651-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-010-0651-y