Abstract
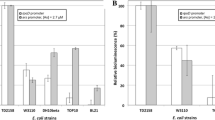
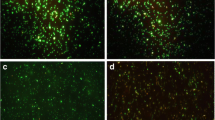
We describe a metal transport system for investigating the interfacial interactions between the anionic surface charge of a gram-negative bacterium (Escherichia coli) and a trivalent cationic metal, Tb3+. We believe this is the first description of the uptake kinetics, sub- and intracellular distribution, and temporal fate of Tb3+ ion in E. coli. We used the luminescence of the terbium–dipicolinic acid chelate to study metal ion transport. The bacteria had a high tolerance for the metal (IC50 = 4 mM Tb3+). Metal ion transport was passive and metabolism independent. The uptake kinetics rapidly reached a maximum within 15 min, followed by a stasis for 60 min, and declining thereafter between 120 and 240 min, resulting in a biphasic curve. During this period, greater than one-third of the metal ion was sequestered within the cell. Our choice of a safe Biosafety Level I E. coli bacteria and the relatively non-toxic Tb3+ metal represents a model system for luminescent investigations of biosorption, for studying bacterial–water interfacial chemistry and for the bioremediation of heavy metals and radionuclides.





Similar content being viewed by others
References
Jiang W, Saxena A, Song B, Ward BB, Beveridge TJ, Myneni SCB (2004) Elucidation of functional groups on gram-positive and gram-negative bacterial surfaces using infrared spectroscopy. Langmuir 20:11433–11442. doi:10.1021/la049043+
Dmitriev B, Toukach F, Ehlers S (2005) Towards a comprehensive view of the bacterial cell wall. Trends Microbiol 13:569–574. doi:10.1016/j.tim.2005.10.001
Rho J-y, Kim J-h (2002) Heavy metal biosorption and its significance to metal tolerance of Streptomycetes. J Microbiol 40:51–54
Pandya S, Yu J, Parker D (2006) Engineering emissive europium and terbium complexes for molecular imaging and sensing. Dalton Trans 23:2757–2766. doi:10.1039/b514637b
Barela TD, Sherry AD (1976) A simple, one-step fluorometric method for determination of nanomolar concentrations of terbium. Anal Biochem 71:351–357. doi:10.1016/S0003-2697(76)80004-8
Brittain HG, Richardson FS, Martin RB (1976) Terbium(III) emission as a probe of calcium(II) binding sites in proteins. J Am Chem Soc 98:8255–8260. doi:10.1021/ja00441a060
Shapiro C, Garcia K, Beller J (1993) Treatment of a simulated mixed waste with supercritical water oxidation. In: Moghissi AA, Blauvert RK, Benda G A, Rothermich NE (eds) Mixed waste, proceedings of the second international symposium, Baltimore, 17–20 Aug 1993, pp 10.3.1.–10.3.16
Andres Y, Thouand G, Boualam M, Mergeay M (2000) Factors influencing the biosorption of gadolinium by micro-organisms and its mobilization from sand. Appl Microbiol Biotechnol 54:262–267. doi:10.1007/s002530000368
Hindle A, Hall EAH (1999) Dipicolinic acid (DPA) assay revisited and appraised for spore detection. Analyst (Lond) 124:1599–1604. doi:10.1039/a906846e
Liu L, Rininsland FH, Wittenburg SK, Achyuthan KE, McBranch DW, Whitten DG (2005) Biocidal activity of a light-absorbing fluorescent conjugated polyelectrolyte. Langmuir 21:10154–10159. doi:10.1021/la046987q
Achyuthan KE (2001) Thiols enhance the sensitivity of luminescent assays for non cross-linked and covalently cross-linked aminophthalhydrazides. Luminescence 16:257–262. doi:10.1002/bio.644
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, pp 58–62
Lippold H, Mansel A, Kupsch HJ (2005) Influence of trivalent electrolytes on the humic colloid-borne transport of contaminant metals: competition and flocculation effects. J Contam Hydrol 76:337–352. doi:10.1016/j.jconhyd.2004.11.005
Ueki T, Sakamoto Y, Yamaguchi N, Michibata H (2003) Bioaccumulation of copper ions by Escherichia coli expressing vanabin genes from the vanadium-rich Ascidian Ascidia sydneiensis samea. Appl Environ Microbiol 69:6442–6446. doi:10.1128/AEM.69.11.6442-6446.2003
Jones GII, Vullev VI (2002) Medium effects on the photophysical properties of terbium(III) complexes with pyridine-2,6-dicarboxylate. Photochem Photobiol Sci 1:925–933. doi:10.1039/b206370k
Jones GII, Vullev VI (2002) Medium effects on the stability of terbium(III) complexes with pyridine-2,6-dicarboxylate. J Phys Chem 106:8213–8222
Ramkumar J (2006) Fluorescence studies of ternary complexes of europium and terbium and their application to analytical determinations. Spectrochim Acta A Mol Biomol Spectrosc 65:992–995. doi:10.1016/j.saa.2006.02.005
Bayer ME, Bayer MH (1991) Lanthanide accumulation in the periplasmic space of Escherichia coli B. J Bacteriol 173:141–149
Beveridge TJ (2005) Bacterial cell wall structure and implications for interactions with metal ions and minerals. J Nucl Radiochem Sci 6:7–10
Beveridge TJ, Koval SF (1981) Binding of metals to cell envelopes of Escherichia coli K-12. Appl Environ Microbiol 42:325–335
Ruming Z, Yi L, Zhixiong X, Ping S, Songsheng Q (2002) Microcalorimetric study of the action of Ce3+ ions on the growth of E. coli. Biol Trace Elem Res 86:165–175
Peng L, Yi L, Zhexue L, Juncheng Z, Jiaxin D, Daiwen P, Ping S, Songsheng Q (2004) Study of the biological effect of La3+ on Escherichia coli by atomic force microscopy. J Inorg Biochem 98:68–72. doi:10.1016/j.jinorgbio.2003.08.012
Oehme I, Wolfbeis OS (1997) Optical sensors for determination of heavy metal ions. Mikrochim Acta 126:177–192. doi:10.1007/BF01242319
Acknowledgments
Sandia is a multi-program laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the United States Department of Energy under contract DE-AC04-94AL85000. Komandoor Achyuthan thanks the National Nuclear Security Administration–Enhanced Surveillance Campaign (NNSA–ESC), the Defense Threat Reduction Agency–Joint Science and Technology Office (DTRA–JSTO) contract no. AA07CBT008, and Sandia’s Laboratory Directed Research and Development (LDRD) project no. 116918 for partially funding these investigations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Achyuthan, K.E., Arango, D.C., Carles, E.L. et al. Luminescent investigations of terbium(III) biosorption as a surrogate for heavy metals and radionuclides. Mol Cell Biochem 327, 87–92 (2009). https://doi.org/10.1007/s11010-009-0046-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0046-0