Abstract
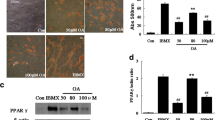
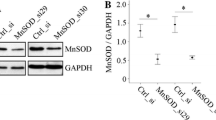
Mitochondrial apparatus is a fundamental aspect in cell, serving for amino acid biosynthesis, fatty acid oxidation (FAO), and ATP production. In this article, we investigated the change of mitochondrial oxidative capacity during porcine adipocyte differentiation and in response to leptin. Rhodamine 123 staining analysis showed about 2-fold increase of mitochondrial membrane electric potential in differentiated adipocyte in comparison with preadipocyte. The mRNA expression of Cytochromes c (Cyt c), carnitine palmitoyltransferase 1 (CPT1), and malate dehydrogenases (MDH) increased markedly (P < 0.05), but that of UCP2 decreased (P < 0.05). Moreover PGC-1α and UCP3 was very low and showed no changes during the adipocyte differentiation. The protein expression of Cyt c and the enzyme activity of Cytochrome c oxidase (COX) increased with preadipocyte differentiation, but cellular ATP level decreased. Furthermore, at the level of 10 and 100 ng/ml leptin not only selectively increased the gene expression of PGC-1α, CPT1, Cyt c, UCP2, and UCP3 (P < 0.05), but also enhanced COX enzyme activity which related to mitochondrial FAO. There is no change of Mitochondrial membrane electric potential and ATP level in cell treated by leptin. These results suggested Mitochondrial is not only critical in FAO, but also play an important role in adipogenesis.








Similar content being viewed by others
Abbreviations
- Cyt c :
-
Cytochromes c
- CEBP/α:
-
CAAT/enhancer binding protein α
- PPAR:
-
Peroxisome proliferator activated receptor
- PGC-1:
-
Peroxisome proliferator-activated receptor γ coactivator
- COX:
-
Cytochrome c oxidase
- CPT1:
-
Carnitine palmitoyltransferase 1
- MDH:
-
Malate dehydrogenases
- UCP:
-
Uncoupling protein
- MRC:
-
Mitochondrial respiratory chain
- NRF:
-
Nuclear respiratory factors
- mtTFA:
-
Mitochondrial transcriptional factor A
References
Sanigorski A, Cameron-Smith D, Lewandowski P et al (2000) Impact of obesity and leptin treatment on adipocyte gene expression in Psammomys obesus. J Endocrinol 164:45–50
Paul T, Chen B, Stuart W (2006) Adipose tissue and adipokines energy regulation from the human perspective. J Nutr 136:1935S–1939S
Audrey C, Maria-Carmen C, Yvette F et al (2004) Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation. J Biol Chem 279:40462–40469
Mercy L, Pauw A de, Payen L et al (2005) Mitochondrial biogenesis in mtDNA-depleted cells involves a Ca2+-dependent pathway and a reduced mitochondrial protein import. FEBS J 272:5031–5055
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78:783–809
Kudo M, Sugawara A, Uruno A et al (2004) Transcription suppression of peroxisome proliferator-activated receptor 2 gene expression by tumor necrosis factor via an inhibition of CCAAT/enhancer-binding protein during the early stage of adipocyte differentiation. Endocrinology 145:4948–4956
Malaga M, Bautista AJ, Salazar JA et al (2000) Lipomatosis, proximal myopathy, and the mitochondrial 8344 mutation. A lipid storage myopathy? Muscle Nerve 23:538–542
Kakuda TN (2000) Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther 22:685–708
Vankoningsloo S, Piens M, Lecocq C et al (2005) Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid-oxidation and glucose. J Lipid Res 46:1133–1149
McKay RM, McKay JP, Avery L et al (2003) C. elegans: a model for exploring the genetics of fat storage. Develop Cell 4:131–142
Rodgers JT, Lerin C, Haas W et al (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118
Lin JD, Wu H, Tarr PT (2002) Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibers. Nature 418:797–801
St-Pierre J, Lin J, Krauss S et al (2003) Bioenergetic analysis of peroxisome proliferator-activated receptor coactivators1a and 1b (PGC-1a and PGC-1b) in muscle cells. J Biol Chem 278:26597–26603
Fruhbeck G, Gomez-Ambrosi J, Salvador J (2001) Leptin-induced lipolysis opposes the tonic inhibition of endogenous adenosine in white adipocytes. FASEB J 15:333–340
Tajima D, Masaki T, Hidaka S et al (2005) Affecting lipolysis and mRNA expression for uncoupling proteins. Exp Biol Med 230:200–206
Gondret F (2001) ADD-1/SREBP-1 is a major determinant of tissue differential lipogenic capacity in mammalian and avian species. J Lipid Res 42:106–113
Li Y, Lu RH, Luo GF et al (2006) Effects of different cryoprotectants on the viability and biological characteristics of porcine preadipocyte. Cryobiology 53:240–247
Stocchi V, Cucchiarini L, Magnani M et al (1985) Simultaneous extraction and reverse-phase high performance liquid chromatographic determination of adenine and pyridine nucleotides in human red blood cells. Anal Biochem 146:118–124
Wilson-Fritch L, Burkart A, Bell G et al (2003) Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol 23:1085–1094
Prunet-Marcassusa B, Moulina K, Carmonab MC et al (1999) Inverse distribution of uncoupling proteins expression and oxidative capacity in mature adipocytes and stromal-vascular fractions of rat white and brown adipose tissues. FEBS Lett 464:184–188
Omatsu-Kanbe M, Inoue K, Yamamoto T et al (2006) Effect of ATP on preadipocyte migration and adipocyte differentiation by activating P2Y receptors in 3T3-L1 cells. Biochem J 393:171–180
Owen OE, Kalhan SC, Hanson RW (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277:30409–30412
Savagner F, Mirebeau D, Jacques C et al (2003) PGC-1-related coactivator and targets are upregulated in thyroid oncocytoma. Biochem Biophys Res Commun 310:779–784
Vianna CR, Huntgeburth M, Coppari R et al (2006) Hypomorphic mutation of PGC-1β causes mitochondrial dysfunction and liver insulin resistance. Cell Metabol 4:453–464
Kamei Y, Ohizumi H, Fujitani Y et al (2003) PPAR γ coactivator 1β/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. PNAS 100:12378–12383
Wagoner B, Hausman DB, Harris RB (2006) Direct and indirect effects of leptin on preadipocyte proliferation and differentiation. Am J Physiol Regul Integr Comp Physiol 290:1557–1564
Park BH, Wang MY, Lee Y et al (2006) Combined leptin actions on adipose tissue and hypothalamus are required to deplete adipocyte fat in lean rats. J Biol Chem 281:40283–40291
Kakuma T, Wang ZW, Pan WT et al (2000) Role of leptin in peroxisome proliferator-activated receptor gamma coactivator-1 expression. Endocrinology 141:4576–4582
Angel A, Desat KS, Halperin ML (1971) Reduction in adipocyte ATP by lipolytic agents: relation to intracellular free fatty acid accumulation. J Lipid Res 12:203–244
Susanne K, Anita S, Stephane B (2001) A effect of the β3-adrenergic agonist Cl316, 243 on functional differentiation of white and brown adipocytes in primary cell culture. Biochim Biophys Acta 1539:85–92
Acknowledgments
This work was supported by the National Basic Research Program of China under Grant number 2004CB117506 and National Natural Science Foundation under Grant number 30471239.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, GF., Yu, TY., Wen, XH. et al. Alteration of mitochondrial oxidative capacity during porcine preadipocyte differentiation and in response to leptin. Mol Cell Biochem 307, 83–91 (2008). https://doi.org/10.1007/s11010-007-9587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-007-9587-2