Abstract
Lactoferrin (Lf) is a glycoprotein belonging to the transferrin family, which can be found in mammalian milk. It was first isolated from bovine milk in the 1930s, and later in the 1960s, it was determined from human milk. This multifunctional protein has the specific ability to bind iron. It plays various biological roles, such as antibacterial, antiviral, antifungal, anti-tumour, anti-obesity, antioxidant, anti-inflammatory and immunomodulatory activities. There are several studies describing its use against in various cancer cell lines (e.g., liver, lung and breast) and the glycoprotein has even been reported to inhibit the development of experimental metastases in mice. Previous studies also suggest Lf-mediated neuroprotection against age-related neurodegenerative diseases and it is also expected to attenuate aging. More recently, Lf has been proposed as a potential approach in COVID-19 prophylaxis. In this review, we discuss the recent developments about the biological activities of this pleiotropic glycoprotein that will reason the exploitation of its biomedical and supplementary nutritional value.
Similar content being viewed by others
Introduction
Lactoferrin (Lf) is a 80 kDa iron-binding glycoprotein of ca. 700 amino acids with high homology among species (González-Chávez et al. 2009). Chemically, it is classified as a member of the transferrin family, proteins that can bind iron ions; in fact, it has a high level of homology with serum transferrin (González-Chávez et al. 2009; Gruden and Poklar Ulrih 2021). Lf consists of simple polypeptide chains with a high degree of glycosylation that are organized and folded into two lobes (N and C lobes) connected by a hinge region containing parts of an α-helix region that confers flexibility to the protein (González-Chávez et al. 2009; Li and Guo 2021).
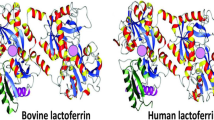
Lf was first found in bovine milk in 1939, then it was isolated in human milk in 1960 (Li and Guo 2021; Elzoghby et al. 2020; Wang et al. 2019). To date, we know that Lf is produced by the epithelial cells of many different mammalian species (e.g., humans, cows, goats, horses, dogs and several rodents) (González-Chávez et al. 2009) with a high degree of homology; in fact, bovine and human lactoferrins (Lfs) have a similarity in structure of about 70%, whereas chimpanzee and human Lfs share nearly 97% (Yount et al. 2007). Human and bovine Lfs have almost the same number of amino acids, i.e., the human Lf has 691 and the bovine Lf has 696 (Baker et al. 2000; Moore, et al. 1997). Figure 1 depicts the similarities in the crystal structure between human and bovine Lfs.
Structures of human lactoferrin (left-hand side) and bovine lactoferrin (right-hand side), depicting the ferric iron (Fe3+) binding sites (purple spheres). (Modified after Kell et al. 2020, Copyright
Lf is not only produced by different species of animals but also by epithelial cells of different body organs (Masson and Heremans 1971); in fact, it is distributed in many types of mammalian secretions such as colostrum, milk, tears, saliva, semen, nasal secretions, bronchial secretions, bile, gastrointestinal fluids, pancreatic secretions urine, vaginal fluids and other mucosal secretions (Masson et al. 1966). Lfs are also produced by the hematopoietic tissue of bone marrow and are found in granules of polymorphonuclear neutrophils (Gruden and Poklar Ulrih 2021; Masson et al. 1966; Berlov et al. 2007).
Lf secondary structure shows both beta-sheet and alpha-helix portions. The single polypeptide chain of lactoferrins is folded into two highly homologous globular lobes that represent the N-terminal and C-terminal halves of the polypeptide. Each lobe, composed of two domains (N1 and N2, C1 and C2), can bind to a ferric ion, and Lf can assume both closed iron-bound (holo) and open iron-free (apo) conformational states. The two lobes are connected by a short, three-turn α-helix (González-Chávez et al. 2009; Li and Guo 2021). Each pair of N-1 and N-2, and C-1 and C-2 domains encloses a deep cleft that provides the iron binding site that is comprised of four amino acids (Tyr [× 2], Asp, His) that provide three negative charges to balance the 3 + charge of Fe3+, together with a helix at their N-terminus and an arginine sidechain, the positive charge of which balances the negative charge on a CO32− anion. Each lactoferrin molecule can bind two iron ions, both reversibly and strongly (K ≈ 1022 M), together with two synergistically bound CO32− ions (Moore et al. 1997; Baker and Baker 2005, 2009). The presence of the carbonate ion appears to play a role in iron release at acid pH (Baker and Baker 2005). This is valid for alpha subtype, which can bind iron and has no ribonuclease activity. While Lf has two others subtypes β and γ that have RNase activity but do not exhibit iron-binding (Gruden and Poklar Ulrih 2021; Li and Guo 2021; Karav et al. 2017).
Lf can exist in many variants due to genetic polymorphism, different post-transcriptional and post-translational processing. Lf is a multifunctional hybrid protein that contains sites of various post-translational modifications, such as phosphorylation, acetylation, ubiquitination or glycosylation among others, affecting its biological function and its chemical and physical properties. Lf exists in several glycosylation isoforms. The number of N-linked glycosylation sites is species specific in fact three potential glycosylation sites have been found in human lactoferrin and five in bovine lactoferrin. Glycosylation plays an important role in regulating LF stability and resistance to proteolysis by increasing the solubility of secreted proteins and increasing the binding of LF to certain cell types or specific receptors whereas it has little effect on properties of LF such as thermal stability or iron binding and release.
The principal isoform is secreted lactoferrin, which has a molecular weight of about 80 kDa, it’s odourless, well soluble in water and shows resistance to heating for several hours at 56 °C while it denatures at 80–90 °C (Gruden and Poklar Ulrih 2021).
It is important for its clinical use to point out that Lf is hydrolysed at the gastric level but this does not render it inactive or unable to perform its functions, in fact scientific evidence and several studies (including clinical ones) (Artym and Zimecki 2013; Chen et al. 2016; Manzoni 2019) suggests that it is hydrolysed to stable, immunologically active peptides upon contact with the acidic environment of the gastric juice. LF can reach the intestine, mainly in the form of peptide fragments (like lactoferricin that is the first peptide derived from the enzymatic hydrolysis of lactoferrin with pepsin) (Gruden and Poklar Ulrih 2021) where they act locally on the microbiota and the immune system associated with the local mucosa. In addition, many studies have shown that lactoferricin is in many cases more active than Lf itself (Gruden and Poklar Ulrih 2021; Kowalczyk 2022).
Lf due to its ability to strongly and reversibly bind iron ions it plays an important role in maintaining iron levels in the body, helping to maintain homeostasis (Baker and Baker 2012; Siqueiros-Cendón et al. 2014a). From the ability to bind iron and the binding constant comes many biological properties of Lf such as anti-inflammatory, antibacterial, reactive oxygen species (ROS) modulator, antiviral, and antitumor immunity effects (Gruden and Poklar Ulrih 2021; Li and Guo 2021; Reseco et al. 2021).
Lf can reduce oxidative stress-induced damage due to its ability to chelate iron ions which is known to have oxidative properties in large amounts, in fact, the presence of high iron concentrations can be toxic: iron can donate electrons to oxygen, forming reactive oxygen species (ROS) as superoxide anions and hydroxyl radicals (Gruden and Poklar Ulrih 2021). Thus, the process of iron chelation by Lf, reduces the generation of free radicals and subsequent organ damage. Lf reduces cytotoxin levels and increases ferric reducing antioxidant power (FRAP) in the intra- and extracellular space. In addition Lf can fight the oxygen explosion in neutrophils which results in extensive production of cell-damaging free radicals (Siqueiros-Cendón et al. 2014a; Lepanto et al. 2019).
In addition, Lf sequesters iron ions necessary for the growth of pathogenic microorganisms, thus also reducing the extent of infectious events. Lf can chelate two iron ions with high affinity at low pH values even around 3, typical of inflamed or infected areas. In addition, Lf showed anti-inflammatory activity against IL-6 and the ability to accelerate the T-cell maturation and differentiation of immature B-cells. That means that Lf controls excess inflammatory response (Gruden and Poklar Ulrih 2021; Rosa et al. 2017).
Lf is also used in the treatment of intestinal disorders as it acts as an antibacterial and prebiotic, on one hand counteracting the infection of pathogens and promoting the proliferation of intestinal microbiota in the digestive tract. It also potentiates the action of antibiotics when co-administered. For this purpose it is often added in nutraceutical products (Hao et al. 2019). Its administration to infants and pregnant women has shown in the former an ability to counter gastrointestinal infections and sepsis, and in the latter a support in the normal development of fetal tissues. Moreover, when administered to pregnant women, it is able to prevent the risk of premature delivery (Kowalczyk 2022; Bukowska-Ośko et al. 2021).
As for infants, Lf has been shown to play a role in immunity in the first few months of life; in fact, those breastfed in the first six months are healthier, thanks to IgG obtained before birth, IgA contained in milk, and Lf boosting the action of antibodies. Fungal infections are also less frequent in children taking Lf (Gruden and Poklar Ulrih 2021; Manzoni et al. 2012). Lf also stimulates the action of vitamin D receptors, proving important in osteogenesis and immunorespons (Li et al. 2021).
Lf also has a role in lipid metabolism, both in counteracting the accumulation of lipids at the level of adipose tissue and reducing their accumulation at the hepatic level (Gruden and Poklar Ulrih 2021).
All the functional properties of lactoferrin depend on its structural integrity. To date we obtain native Lf by isolating it from milk and colostrum (or other secretions) or as recombinant Lf from bacterial, fungal and viral expression systems (González-Chávez et al. 2009). Because Lf is sensitive to high temperatures and other chemical and physical stresses that can denature it, optimizing the extraction of native Lf and powder formation processes is recommended so that structural damage can be reduced (González-Chávez et al. 2009; Wang et al. 2019).
To highlight the relevance of this glycoprotein and its biological and nutritional values, the bibliometric map was generated by the VOSviewer software (Eck and Waltman 2010, 2022), using the combination of terms “Lactoferrin” AND “Biological activities” AND “nutritional” as keywords. A total of 1547 articles were listed, out of which 70 only in 2023. The outputs of the search run on the Scopus database are shown in Fig. 2, fine-tuning the search only to articles published between 2020 and 2023, within the following disciplines: (i) agricultural and biological sciences; (ii) biochemistry, genetics and molecular biology; (iii) medicine; (iv) immunology and microbiology; and (v) pharmacology, toxicology and pharmaceutics, downsizing the list to 606 articles.
Bibliometric map obtained by VOSviewer software version 1.6.16 (https://www.vosviewer.com) (Eck and Waltman 2022), using Lactoferrin” AND “Biological activities” AND “nutritional” as searched terms, registered from Scopus database (3rd April 2023)
From the outputs shown in Fig. 2 (terms were searched in articles’ abstract and keywords), 621 co-occurrences were recorded, clustered into four categories namely, 194 items for the red cluster (e.g., bioavailability, peptide, peptides, functional food, chemistry), 187 items for the green cluster (e.g., animals, metabolism, intestinal flora, nutrition), 154 items for the blue cluster (e.g., drug delivery systems, oxidative stress, drug effect flavonoid), and 86 items for the yellow cluster (e.g., in vivo study, enzyme activity, anti-bacterial agents, corona virus).
Lactoferrin for Antibacterial, Antiviral and Antifungal Treatments
Antibacterial Activity
Lf showed activity against a large spectrum of pathogenic microorganisms, proving to be a protein with antibacterial (against gram-positive and negative bacteria), antiviral, and antifungal properties. It also has activity against protozoa (Orsi 2004). Lf has also proven to be an interesting molecule in drug research against multidrug-resistant strains of bacteria. To counter bacterial strains' resistance to commercial drugs, research has turned to innate immune system proteins such as Lf, for which no bacterial resistance has been observed (Avalos-Gómez et al. 2022).
Lf has mainly two mechanisms of action underlying its antibacterial activity (against both gram-negative and positive bacteria), one bacteriostatic and one bactericidal (iron-independent). Lf showed bacteriostatic activity due to its ability to sequester iron by taking it away from the bacteria that need it for their growth and proliferation. Addition of exogenous iron beyond the binding capacity of Lf leads to resumption of bacterial growth. This confirms the role of the ion (Arnold et al. 1982). Moreover, Lf has a direct bactericidal action due to direct interaction with the microorganism and in particular due to its ability to bind to the lipid A constituting LPS of the bacterial cell membrane by increasing its permeability (Gruden and Poklar Ulrih 2021). Membrane LPS release increases the permeability and susceptibility of bacteria to the action of lysosomes, which are important in the immune response against pathogens (Gruden and Poklar Ulrih 2021; Al-Mogbel et al. 2021). This bactericidal action is mainly operated by lactoferricin, the first peptide derived from Lf by enzymatic hydrolysis that has shown not only antibacterial but also antiviral and antifungal activity.
Other mechanisms underlying the antibacterial activity of Lf have also been described such as inhibition of adhesion and colonization, thus preventing biofilm development; but also induced apoptosis in infected cells and bactericidal activity of PMNs (Orsi 2004). Lf is able to inhibit or kill bacterial cells either by direct action on them but also by stimulating the immune system, the synthesis of cytokines and chemokines or by accelerating the maturation of immune system cells. Moreover, at the intestinal level it increases tight junction production by increasing the tightness of the intestinal epithelium (Gruden and Poklar Ulrih 2021; Artym et al. 2018; Siqueiros-Cendón et al. 2014b).
The direct iron-independent bactericidal action of Lf was discovered in 1977 when it was observed that human Lf inhibited the growth of Vibrio cholerae and Streptococcus mutans (upon contact with an iron-rich medium), but that this bacteriostatic activity did not regress upon addition of iron. By immunofluorescence techniques, the binding of Lf to the bacterial cell surface has been observed, and to date we know from several studies that Lf binds to surface of bacterial cells and causes the release of LPS from the wall of gram-negative bacteria thus interacting directly with the membrane (Lizzi et al. 2016).
Lf is capable to bind LPS only in the apo-Lf conformation when it does not bind iron (the form in which it is physiologically present in fluids). Iron-binding holo-Lf does not inhibit bacterial growth (Lizzi et al. 2016). This is because Lf interacts with the lipid A of LPS via an essential sequence of four arginine present on the N-terminal domain loop region that undergoes conformational changes based on whether or not it binds to iron. In silico studies have shown that apo-Lf is in a more open conformation than holo-Lf, and this allows it a better interaction between the N-terminal domain and the bacterial membrane (Lizzi et al. 2016; Berkel 1997).
Furthermore, the release of LPS from the wall of gram-negative bacteria and subsequent wall flaking can be blocked by high levels of divalent cations such as Ca2+ and Mg2+. This is again due to conformational changes in the protein, which in the presence of Ca2+ can undergo polymerization to form tetramers. In addition, the negative charge of the head of the LPS molecule binds with an electrostatic interaction to divalent cations, thus stabilize the structure of LPS and the bacterial wall’s integrity, protecting pathogens from the antibacterial action of Lf (Gruden and Poklar Ulrih 2021; Bennett et al. 1981). In fact, the addition of chelators such as EDTA, which sequester bivalent cations, prevent membrane stabilization by them, and the release of LPS from the wall is observed (Nikaido and Vaara 1985).
The antibacterial action of Lf also depends on pH values, temperature, and the presence or absence of buffers. Lf shows higher activity at temperature of 37 °C and acidic pH between 5.0 and 6.0 (Gruden and Poklar Ulrih 2021; Kalmar and Arnold 1988).
Lf is also able to bind porins, a transmembrane channel protein present on the wall of bacteria that pemits the nonselective diffusion of hydrophilic solutes through the wall (Gruden and Poklar Ulrih 2021; Nikaido 2003).
Lf is also able to downregulate the luxS gene, which codes for a protein critical in biofilm formation, preventing its formation (Gruden and Poklar Ulrih 2021; Al-Mogbel et al. 2021). Lf has been shown to have anti-adhesive properties against bacterial strains in the respiratory and intestinal mucosa (Araújo and Giugliano 2001; Kawasaki et al. 2000). The N-glycans in human Lf prevent the adhesion of Salmonella enterica typhimurium, Salmonella enterica enteridis, monocytogenes and Pseudomonas aeruginosa. Furthermore, it has been shown that those responsible for the antibacterial activity of lactoferrin are the sialylated glycans; the neutral ones have no activity (Gruden and Poklar Ulrih 2021; Kautto et al. 2016; Karav 2018).
Lf can also reduce glucose uptake and inhibit lactic acid synthesis, thus affecting glucose metabolism. It has a synergistic action with bacteriophages, some antibiotics, and with lysosomes and their enzyme array (critical in the action of PMN lymphocytes) (Al-Mogbel et al. 2021; Zimecki, et al. 2008).
It has been shown that Lf is also active following hydrolysis. Indeed, it has been noted that following exposure to acidic conditions (pH 2–3) and high temperatures (100–120 °C), antibacterial activity is maintained and sometimes enhanced (Saito et al. 1991). In fact, through Lf’s enzymatic hydrolysis with pepsin, we obtain peptides able to inhibit the growth of gram-positive and negative bacteria, including some strains resistant to Lf itself (Tomita et al. 1991). The first peptide obtained from the enzymatic hydrolysis of Lf is lactoferricin, which has shown better antibacterial properties than Lf. In addition, in vivo and in vitro studies have shown that Lactoferricin is formed spontaneously in the body following gastric degradation of Lf and that bovine lactoferricin has higher activity than those of other mammals (Furlund et al. 2013). Studies have also shown that even smaller fragments of lactoferricin have antibacterial activity. The portion of the peptide essential for antibacterial activity is a region composed of 11 amino acids, between 20 and 30 in bovine Lf (Gruden and Poklar Ulrih 2021; Liu et al. 2011).
Moreover, bovine lactoferricin showed better antibacterial activity when it assumes the cyclic structural conformation than the linear one (Vorland et al. 1998). This can be explained by better penetration of the cyclic form into the bilayer of the bacterial membrane and even by more stable bonds’ formation with the negatively charged portion of the membrane (Mika et al. 2011). The active portions of Lf and lactoferricin are those that have beta-sheet conformation in the presence of LPS. Therefore, the beta-sheet structure of lactoferricin is more effective in initial contact with the bacterial membrane than the helical structure of native Lf (Gruden and Poklar Ulrih 2021; Chapple et al. 2004; Farnaud et al. 2004a).
As already mentioned, bovine lactoferricin has better activity than human lactoferricin, this could be due to the presence of two tryptophan residues in the loop region of bovine lactoferricin, compared to human lactoferricin which has only one. In fact, it has been shown how the aromatic character and the shape of the tryptophan residues influence antibacterial activity. In addition, the substitution of basic and hydrophobic amino acids in the structure of Lf-derived peptides results in the loss of antibacterial activity. This demonstrates their importance (Gruden and Poklar Ulrih 2021).
The mechanisms of action of lactoferricin are partly common to those of lactoferricin; in fact, lactoferricin due to its amphiphilic structure involving a hydrophobic region lined by hydrophilic and cationic amino acids, is able to interact with the anionic surface of the bacterial membrane causing the release of its constituent LPS. Lactoferricin has also been shown to interact selectively with bacterial cells rather than eukaryotic cells (Gruden and Poklar Ulrih 2021; Chapple et al. 2004; Farnaud et al. 2004b). Additionally, lactoferricin can also form pores in phospholipid membranes, and the absence of cell lysis suggests that lactoferricin can also penetrate cells and damage them by interfering with intracellular mechanisms. In fact, some studies have shown how lactoferricin is able to inhibit the synthesis of bacterial proteins and how some of its derivatives are able to bind genomic DNA, to inhibit synthesis not only of proteins but also of DNA and RNA, and to inhibit the phosphorylation of the response regulators BasR and CreB (Ho et al. 2012). As seen for lactoferrin, the antibacterial activity of lactoferricin is also pH dependent and reduced by the presence of divalent cations such as Ca2+, Mg2+ or monovalent such as Na+ and K+ (Gruden and Poklar Ulrih 2021).
Antiviral Properties
Lf has antiviral activity against enveloped or naked viruses, and prevents infection in both humans and animals. Its antiviral activity is due to the synergistic action against some antiviral drugs such as acyclovir, ribavirin or zidovudine (Berlutti et al. 2011), but it also plays a role in the early stages of viral infection, preventing the virus from entering host cells either by binding directly to the surface of the virion (by binding to virus envelope proteins) or by binding and blocking the cellular receptors that would be used by the virus to adhere to it and entry into host cells (Gruden and Poklar Ulrih 2021; Strate et al. 2001; Superti et al. 2019; Drobni et al. 2004). In fact, Lf has antiviral activity for example against SARS-CpV-2 as it binds to the ACEII receptor present on cells which is the same used by the virus for anchorage. By blocking the receptor, Lf prevents the virus from entering the cells. Lf is also able to prevent virus-induced cell apoptosis and influence other intracellular mechanisms (Pietrantoni et al. 2010; Ammendolia et al. 2007). Unlike what is shown for the antibacterial activity, the antiviral activity is greater for the native form of Lf, even if it’s been observed also for the derived peptides (Andersen et al. 2003).
Lf has action against the influenza A virus as it is able to prevent the action of caspase3, a protease that plays a crucial role in regulating apoptosis. In this way Lf controls the apoptosis of infected cells. Moreover, studies have revealed how Lf is able to block the replication of the virus and, in particular, to block the assembly of new virions, blocking the nuclear transport of ribonucleoproteins. Moreover, Lf can interact through the C-lobe with the HA2 fragment of hemagglutinin, a glycoprotein present on the envelope of the virus that has a fundamental role in allowing binding to cells and penetration of the virus (Gruden and Poklar Ulrih 2021).
Human and bovine lactoferrin have been shown to prevent infection of lymphocytes and hepatocytes by HCV virus (etiologic agent of cirrhosis and hepatocellular carcinoma) through a direct interaction with the virus itself (Hara et al. 2002). In particular, a region of bovine lactoferrin formed from amino acids 600–692 called C-s3 is able to interact with viral glycoproteins E1 (fusogenic, which facilitates virus fusion with cells) and E2 (important in virus entry). In particular, Cys628 is responsible for glycoprotein binding and thus activity. The same mechanism of action has been shown against HBV (Gruden and Poklar Ulrih 2021).
Lf also plays a role in preventing infection of herpes simplex viruses 1(HSV1) and herpes simplex viruses 2(HSV2) by binding to heparan sulfate and chondroitin sulfate glycosaminoglycans present on cell membranes. Thus, Lf acts in the early stages of infection. In fact, through immunofluorescence assays it has been shown that Lf is arranged on the surface, but it is also visible how Lf is distributed in the cytoplasm and intracellular spaces. In fact, Lf and lactoferricin interfere with protein trafficking through the nucleus, preventing virus replication and assembly (Gruden and Poklar Ulrih 2021; Andersen et al. 2004).
Lf and lactoferricin also have activity against HIV1 and HIV2 due to their globular structure and negative charge (characteristics required for antiviral action against these viruses). Again, Lf interacts with viral replication probably by preventing adhesion and entry of the virus into cells. Lf has been shown to bind the V3 loop of the gp120 protein on the HIV envelope with high affinity, but the precise mechanism of action of the anti-HIV action is not yet known with certainty. The action of Lf in interfering with HIV-1 reverse transcriptase, protease and integrase, three enzymes essential for the survival of the virus, was also evaluated (Gruden and Poklar Ulrih 2021; Berkhout et al. 2002).
Lf has shown contrasting activities in relation to adenoviruses (naked viruses). On the one hand they have shown antiviral activity through two different mechanisms of action: Lf can bind to cell wall glycosaminoglycans, preventing viral infection; it can also bind to polypeptide III and polypeptide IIIa on viral particles, affecting replication. On the other hand Lf has been seen to promote virus adhesion to epithelial cells and enhance infection in dendritic cells (Johansson et al. 2007). Notably, only the N lobe showed cytotoxic action against adenoviruses (Pietrantoni et al. 2003).
Lf both human and bovine showed antiviral activity against α-papillomavirus HPV-16 and β-papillomavirus HPV-5. The bovine one shown a greater activity (Drobni et al. 2004). Regarding lactoferricin, only the circular one (among those tested) showed antiviral activity against HPV (Mistry et al. 2007). Other studies have demonstrated an action of Lf in preventing viral infection by rotavirus (non-enveloped virus) and echovirus. Lf has shown an action in restoring virus adhesion to cells, here of in the early stages of infection, but also in the later stages (Gruden and Poklar Ulrih 2021; Tinari et al. 2005).
Regarding antiviral action one can find many studies in the literature dating back to the 2000s (Strate et al. 2001). The most recent ones mainly refer Sars-Cov-2 and some new results obtained. For example, in vitro studies have shown how camel Lf can block HCV infection in cell culture at a concentration of 0.25 mg/mL. Moreover, Lf pre-treated cells with 1 mg/mL and then infected with HBV have shown the same result and breast milk extracts at the concentration of 10 mg/mL has an inhibitory efficacy between 80 and 90%. Lf can in fact prevent the infection in the first part of the viral cycle by binding directly the hepatitis B antigen surface(Jose-Abrego, et al. 2022).
In a recent study, the action of Lf against Enterovirus E (EV-E) that is a member of the picornaviridae family was investigated. Usually EV-E causes subclinical infections but in some conditions can lead to serious complications in the respiratory, digestive, and reproductive systems. There is no specific cure but only systemic treatment. In the study, Lf was shown to inhibit virus replication during uptake and at the next stage. Other studies reported to decrease viral RNA levels in the cell by up to 75% (Wróbel et al. 2022).
In an in vitro study conducted in Japan, Lf was shown to reduce norovirus infection. Noroviruses cause morbidity and mortality worldwide and there are not still approved antiviral treatments or vaccines. The study was conducted on a B cell line with an indirect mechanism, through induction of the innate interferon response (Oda et al. 2021).
Lf Against Sars-Cov
Lf also showed antiviral action against SARS-COV (an RNA-coated virus) that infects cells through the interaction between the spike protein on its own wall and the ACEII (angiotensin-converting enzyme 2) on host cells. Various mechanisms of action of Lf have been proposed including an interaction with the spike protein, increased interferon response (Mirabelli et al. 2021), and interaction with heparan sulfate proteoglycans (HSPG) on host cells (it has been shown by some studies that human coronavirus requires heparan sulfate for infection) (Campione et al. 2021; Hu et al. 2021; Milewska et al. 2014).
Coronaviruses have received a lot of attention in recent times since they have strained global health. Sars-Cov-2 is the coronavirus that has caused the latest global pandemic as of 2019 and for which we have more recent data for that reason, but other coronaviruses that have caused problems for global health are SARS-CoV-1 and MERS-CoV and 4 other very common coronaviruses, responsible to 15–30% of cold cases worldwide (Enjuanes et al. 2016; Andreu et al. 2023).
Coronaviruses belong to the family coronaviridae, whose Latin-derived name alludes to the presence of a crown (“corona” means “crown”), which is actually represented by the presence of spike protein coating the surface of the virion (Mahendra and Kavitha 2021). Coronaviruses can be classified into 4 genera: α, β, δ, γ. The first two genera infect mammals, gamma-type infect avian species, and delta both. SARS-CoV, MERS-CoV, and SARS-CoV-2 are beta-type (Bolat et al. 2022; Shereen et al. 2020). SARS-Cov are positive-strand RNA viruses, with envelope. Indeed, they have spike proteins, envelope, membrane and nucleocapsid proteins.
Infection of virions to the host cell takes advantage of the initial processes of adhesion of the virus surface to the cell. In the case of Sars-Cov-1 and 2, it has been observed that these viruses adhere to the cell via interaction between the spike protein and the ACEII receptor present on cells of epithelia such as respiratory but also intestinal (Ahlawat and Sharma 2020; Dai, et al. 2020). Specifically the alpha1 receptor-binding domain (RBD) present on the virus interacts via a polar interaction with peptidase domain (PD) of ACE2 (Yan et al. 2020).
Additionally, another binding point that facilitates virus adhesion to the cell wall is the binding between the virus and heparan sulfate proteoglycans HSPGs that are distributed on the cell surface. Binding between the virus and HSPGs is an initial contact that allows the accumulation of viral particles on the cell and then allows binding to ACEII and penetration. In fact, after binding HSPGs the virus rolls onto the surface and penetrates the cell. An additional independent receptor identified for Sars-Cov-2 is a dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN).
These virus binding sites to cells are useful in understanding the mechanisms of action of Lactoferrin in the early stages of infection. It has been shown that Lf can bind to HSPGs by occupying an anchor site for the virus, and thus preventing infection early in the process (Wang et al. 2020). Indeed, it prevents the initial adhesion of the virus to the cell and thus also the binding to ACEII (Pasquale et al. 2021; Einerhand, et al. 2022). In fact, even though early studies had initially found no interactions between Lf and ACEII, computational studies have attempted to investigate with respect to Lf's ability to interact with the ACEII receptor and/or the RBD domain of the virus’ spike. These studies have in fact identified portions of ACEII complementary to Lf (Miotto et al. 2021a). In addition, in in vitro studies they investigated the affinity between ACEII, the RBD domain of the spike, and Lf by using two different independent methods (biolayer interferometry and latex nanoparticle-enhanced turbidimetry) investigating both kinetic and thermodynamic parameters and pairwise interactions. From the results obtained, it is clear that Lf knows how to bind ACEII with high affinity at its ectodomain, but not only that, at a concentration of 1 μM it knows how to interfere with the formation of the RBD-ACEII complex, leading to a 300-fold increase in the relative KD of the complex itself. Whereas, on the contrary, no interactions between Lf and RBD are observed at the highest physiological concentrations of Lf. Thus, the data lead to the conclusion that the inhibitory action of Lf is due to binding with ACEII (Piacentini et al. 2022).
However, Lf appears to interact with the viral surface (Miotto et al. 2021a). In fact, there are also studies demonstrating the link between Lf and the coronavirus spike protein. There are also hypotheses that DC-SIGN can promote ACEII-mediated infection and a study carried out on dengue virus showed that Lf knows how to bind to various sites on the membrane including HS and DC-SIGN, it is possible that Lf knows how to indirectly inhibit ACEII-mediated infection, interacting with virus surface, in particular with DC-SIGN (Serrano et al. 2020).
In addition, Lf has another mechanism of action. Lf, in fact, by interacting with its receptor on the cell surface is able to increase production in alpha and beta interferon (IFN), inhibiting viral replication even once virus penetration into the cell has occurred. In fact, Lf can prevent the viral replication also due to its ability to inhibit viral RNA-dependent polymerase, helicase and 3CL proteases that are crucial for the normal replication cycle of the virus. Lf also shown its ability to increase the expression levels of genes related to the immune response of the cell against viral infection thus stopping the infection (Kaczyńska et al. 2023).
Lf also because of its ability to bind iron is able to reduce the oxidative stress (and the consequent cell damage) caused by the virus. The virus, in fact, can attack hemoglobin leading to a release of iron and oxygen that react and result in ROS. Thus, Lf can counteract the damage and the consequences of virus action. It was demonstrated by in vitro tests on a human embryonic diploid fibroblast cell line (MRC5) using 2′,7′-dichlorodihydro-fluorescein diacetate (H2DCF-D) that Lf has the ability to inhibit ROS formation in a dose-dependent manner (Zimecki et al. 2021).
We also know that high Ferritin levels (due to the virus infection) correlate with increased inflammatory phenomenon, cause cytokine storm, including IL-6. This causes dysregulation in iron homeostasis levels leading to over-accumulation of iron in the cell. This promotes viral replication since some enzymes involved in transcription, translation, and assembly of new virions are iron-enzymes. It has been seen in patients with COVID-19 that Lf is able to reduce ferritin and IL-6 levels and chelating iron is able to reduce intracellular overload and thus reduce viral replication.
COIVD-19 induces a reduction of B-cells (important in defense) and causes a massive release of cytokines (cytokine storm). In fact, a hyper-stimulation of immune system leading to a large release of pro-inflammatory cytokines by neutrophils, T-helper, and T-cytotoxic cells. In addition, Sars-Cov-2 induces an increase in coagulation and, as already mentioned, causes damage to haemoglobin by dysregulating iron levels.
Lf also has a role in regulating both innate and adaptive immune response due to its positively charged N-terminal domain with which it can bind to the cell surface and influence the expression of pro- and anti-inflammatory cytokines. Lf can be internalized in host cell by binding the transferrin receptor and once up-taken, can enhance the expression of antiviral genes and decrease the expression of pro-inflammatory cytokines (Ward et al. 2022). Lf in fact causes a decrease in the levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and the maintenance or increase of anti-inflammatory cytokines (IL-10 and TGF-β) (Einerhand et al. 2022). Lf can contain the release of IL-6, TNF-alpha, and increase the release of IL-10. Was indeed demonstrated in a model of pulmonary acute respiratory distress syndrome (ARDS) in granulomatous inflammation that Lf reduces lung tissue damage, profiled by cytokine storm (Einerhand et al. 2022; Mrityunjaya et al. 2020). Lf, as demonstrated by several studies, promotes maturation of immature B-cells to APCs (antigen presenting cells) and increase the total T-cell activation (Einerhand et al. 2022).
The fact that Lf is able to control cytokine storm is important in reducing mortality. In fact, these pro-inflammatory molecules have many receptors on platelets. Over-stimulation of these receptors leads to thrombocytopenia (low platelet count) and the risk of hypercoagulation, which are linked to an increased risk of mortality in COVID-19 patients (Kell et al. 2020). However, some recent clinical trials tried to demonstrate the ability of Lf to reduce mortality in severe patients but they did not find out substantial differences when analysing different parameters in the group of treated versus untreated patients. Nevertheless, Lf shown a good safety profile and tolerability. More studies are needed (Matino et al. 2023; Navarro et al. 2023).
Again, in trying to counter COVID-19, it has been seen that an improved lifestyle can reduce inflammatory phenomena and cytokine levels. Through physical activity and a diet rich in certain nutrients, it has been possible to reduce the symptoms of the condition without the use of drugs and thus avoiding attached side effects. One example could be Lf itself as a nutritional supplement which can reduce inflammation, balance immune system and restore the health of the barrier microbiota. The fact that Lf knows how to maintain a healthy salivary and intestinal microbiota is useful both because the oral cavity plays an important role in infection and virus transmission, and in the case of bacterial co-infections or secondary infections that may occur during the hospitalisation of critically ill patients. Indeed, Lf knows how to inhibit the growth of pathogenic bacteria but preserve the viability of physiological ones. Figure 3 schematically represents the main Lf action against the virus, whereas Table 1 summarizes distinct mechanisms of action of Lf against Sars-Cov-2 numbered in Fig. 3, with reference to in vitro and in vivo studies, and clinical trials.
Schematic representation of antiviral actions of Lf against SARS-CoV-2 (Mechanisms of action identified as 1–4 are referenced in Table 1
Antifungal Properties
Lf has also shown a dose-dependent antifungal action, both due to the fact that it is able to act synergistically with antifungal drugs and because it has action against dermatophytes. Studies have demonstrated that bovine lactoferrin reduces the incidence of invasive fungal infection in preterm very low birth weight (VLBW) neonates as a result of limitations in the ability of fungal colonies to advance towards invasion and systemic disease in colonized infants. Antifungal action has been recorded against candida albicans and also against candida krusei. It has been seen that, as with antibacterial activity, the action is affected by pH, temperature, and the presence of ions. In particular, the presence of bivalent cations like Ca2+ and Mg2+, reduces the antifungal properties. Lf binds directly to the cell membranes of the fungus with destructive consequences. It has been seen that high concentrations of iron (enough to saturate Lf) cause loss of antifungal activity (Gruden and Poklar Ulrih 2021).
Lf and its derivatives bind on the surface of G. lambia with giardicidal action even at low concentrations. At low concentrations they caused dilatation of the endoplasmic reticulum (ER) membranes, expansion of the nuclear membrane and protrusions of the plasmic membrane. While at high concentrations they cause alterations in cell morphology and can induce programmed cell death (Aguilar-Diaz et al. 2017).
Because many microorganisms need iron to grow and proliferate, the scavenger ability of the glycoprotein is effective in limiting their vitality. However, some parasites (like Trypanosoma brucei, Trichomonas vaginalis, Trichomonas foetus and Leishmania chagasi) that require high concentrations of iron to live, have learned to acquire iron from external sources, including proteins such as transferrin and lactoferrin. For T. Goondii, Lf does not inhibit penetration into cells and has no direct cytotoxic action but inhibits the multiplication of protozoa (Gruden and Poklar Ulrih 2021; Dzitko et al. 2007).
In particular concerning Candida species, these are the most common cause of fungal opportunistic pathogens and source of systemic infection associated to drug resistance. They are able to form biofilm on medical devices. There are several in vitro and in vivo studies about the antifungal action of Lf against Candida since the consequences of the infection are common amongst the population. Candida albicans is in fact responsible of recurrent vulvovaginal candidiasis (VVC) in childbearing women. In a study conducted in 2019, the antifungal properties of bovin Lf were tested both in vitro and in vivo. In particular, the researchers constructed a strain of Lactobacillus casei called L.casei/pPG612.1-BLF capable of secreting BLF by encoding it from a plasmid vector pPG612.1. L. casei/pPG612.1-BLF in contact with Candida albicans colonies in a two-layer agar plate in vitro assay has been shown to reduce the number of CFUs and reduce their average size. Furthermore, in the same study che strain was tested in an in vivo mouse model; it was found that the severity of the infection was lower in mice pre-treated with L.casei/pPG612.1-BLF intra-vaginally than in the control. Furthermore, the severity of the infection was reduced in mice that were treated continuously for 5 days with topical L.casei/pPG612.1-BLF. Therefore, the compound tested showed both a preventive and curative action and demonstrated the ability of L. casei to be probiotic but at the same time a vehicle for therapy (Liao et al. 2019).
In another study conducted in the same year, it was shown that the oral administration of Lf and probiotic bacterial strains (Lactobacillus acidophilus GLA-14, Lactobacillus rhamnosus HN001) concomitant with the topical administration of Clotrimazole is important in reducing the recurrence of infection. Forty-eight women with symptoms of VVC, positive for candida infection, and with past histories of recurrence were divided into two groups: one subjected to placebo and one to verum, both treated with Clotrimazole. Both groups showed improvement in symptoms. However, only the verum group (treated with Lf and prebiotics) showed a reduction in itching and discharge at 3 and 6 months. Moreover, recurrences during the 6-month follow-up were significantly lower in the group of treated women compared with placebo (33.3% vs 91.7% after 3 months and 29.2% vs 100% after 6 months) (Russo et al. 2019).
This is a very important aspect of trying to reduce drug resistance as well as alleviating symptoms and reducing the frequency of infection. In fact, the widespread and frequent use of azoles as first-line treatment in treating fungal infections led to the development of drug-resistant strains.
Candida strain and microbial imbalance in oral environment can also cause oral and esophageal candidiasis. An in vivo study in immunocompromised mice with oral candida showed that administration of bLf in the water they drank significantly reduced damage to the tongue mucosa and reduced the number of fungal cells (Krupińska and Bogucki 2021). In an another in vivo study in an animal model of ltf-/- mice, administration of exogenous Lf showed a reduction in CFUs in oral tissue and several organs in both ltf-/- and wild type mice compared with the untreated control group. A decrease in the expression of genes responsible for Candida virulence was observed in the treated mice. Moreover, in the gingival tissue of treated animals, there was increased expression of various interleukins, TNF-α, TGF-β and other mediators, compared with untreated tissue (Pawar et al. 2022).
Furthermore, in an HIV-infected patient with chronic and relapsing oral candida, the use of an antifungal in combination with a lysozyme and Lf mouthwash resulted in complete resolution of the infection. A tissue conditioner containing a cation exchange resin capable of increasing the concentration of Lf in the oral cavity was also developed in a research study. This was found to be useful in preventing and treating denture stomatitis and opportunistic infections. Lf is currently contained in many oral care products, such as toothpastes, mouthwashes, denture adhesives (Krupińska and Bogucki 2021).
As mentioned earlier, one of the current challenges is to cope with drug resistance. One of the strategies implemented and possible is to create combinations. A study in 2022 showed that the combining use of caspofungin (conventional antifungal) and Lf was able to restore caspofungin sensitivity in candida strains resistant to this drug, both in free cells and in cells organized in biofilms (Fais et al. 2022).
Lactoferrin for Aging-Related Neurodegenerative Diseases
According to recent reports, Lf has a role in the treatment of aging-related neurodegenerative diseases, as well as the treatment of stress-related emotional disorders (Kowalczyk 2022; Li et al. 2022; Guzmán-Mejía et al. 2021). Aging is a natural process of life in which, because of genetic and environmental factors, cells, tissues, organs and systems reduce their function (Tian et al. 2022; López-Otín et al. 2013). This function deterioration leads to the risk of pathologies development (Reseco et al. 2021). Lf has an anti-aging effect because of its many pharmacological functions: such as anti-cellular senescence, anti-inflammation, anti-carcinogenic and antioxidant. In addition, Lf can modulate the signal pathways involved in the longevity of the organisms (Li et al. 2022).
The Global public health has recently increased its efforts for reducing the risk of aging-related diseases (including also neurodegenerative diseases). For this purpose, it is necessary to conduct research on compounds that modulate the function of the neuroendocrine pathways, because these pathways are responsible for diseases related to stressful situation. In fact, when the organism goes through a stressful situation, the neuroendocrine pathways (which include a lot of chemicals mediators) can activate or worsen different pathological states, such as all the aging-related diseases (Li et al. 2022; Guzmán-Mejía et al. 2021).
Neurodegenerative and aging-related diseases are associated with the central nervous system (CNS). Among these pathologies we include Parkinson’s disease (PD), Alzheimer’s disease (AD), Down’s syndrome, cerebrovascular disease, amyotrophic lateral sclerosis and other neurological disorders. Since most of CNS diseases are related to high oxidative stress levels and high inflammatory response, Lf is associated with these diseases for its anti-inflammatory, ROS modulator and anti-apoptotic functions. In fact, previous studies have confirmed the over expression of Lf in many neurological and neurodegenerative diseases. However, even if lot of studies about this topic have been done, the knowledge about Lf’s mechanism of action in CNS diseases, especially in neurogenesis regulation, is still limited (Li and Guo 2021).
It has been verified that in patients with neurodegenerative diseases is the leak of neurons and cells either in the brain or in the spinal cord, and it is also known that the consequences of these diseases are behavioural and cognitive imbalance or even death in severe cases. Between all the neurodegenerative disease, AD and PD are relatively well-known. From the studies about these two diseases, it was possible to understand that neuroinflammation, oxidative stress, lipid metabolism dysregulation, iron disorders and other transitional metals disorders are mainly included in neurodegenerative diseases pathogenesis (Li and Guo 2021). Since high levels of Lf were found in damaged areas and inflamed tissues of different neurodegenerative diseases, many studies has tried to associate the Lf to the mechanisms involved in neurodegenerative diseases pathogenesis: such as down-regulation of pro-inflammatory cytokines (TNFα, IL-6 and IL-1β) expression that leads to an anti-inflammatory effect (Spagnuolo and Hoffman-Goetz 2008); up-regulation of superoxide dismutase (SOD) that inhibits the Fenton reaction and leads to a reduction of ROS levels (Trentini et al. 2020); regulation of lipid metabolism through the control of the function of cAMP/ERK (extracellular regulatory protein kinase) signalling pathway via LRP1 (Ikoma-Seki et al. 2015); seizure of ionic iron necessary in microbial growth, in order to improve host immunity defence against infections (Reseco et al. 2021).
Several studies were done on the importance of iron ion in aging. In fact, it has been shown that in aging cells there is a high iron ion accumulation, which leads to a DNA damage that cause the acceleration of the aging process, this is called Ferrosenescence. The main problem with iron accumulation is that, through the Fenton reaction, it produces high levels of ROS, which cause different damages to macromolecules that lead to the cell death. Especially when the iron accumulates in the brain, it leads to lot of neurodegenerative diseases. To solve this problem, it is possible to use many iron chelators, which can reduce the iron levels. Lf has chelating properties because it binds iron and reduce the iron accumulation. Despite this, iron chelators are useful only if iron accumulation is the cause of aging; if iron excess is the consequence of aging, it does not have much sense to use iron chelators. It is necessary to keep investigating on this point (Tian et al. 2022).
It has been shown that, through transcytosis mediated by specific receptors, Lf can penetrate into the brain parenchyma (Kopaeva et al. 2019). For this reason, many studies tried to administrate exogenous Lf in induced animal models of neurodegenerative diseases. The first example concerns Lf administration in aged mice, which leads to improvement of spatial cognition thanks to inflammation and oxidative stress reduction in the hippocampus (Zheng et al. 2020). The second example concerns the decrease of motor deficits in induced mouse model of PD (Liu et al. 2020) and the third example is a study on transgenic mouse model of AD administered with Lf: they showed a lower aggregation of amyloid-beta (aβ), which leads to improvement of cognitive performance (Abdelhamid et al. 2020). Probably one of the main reasons why Lf helps in PD and AD is its accumulation in dopamine neurons for PD cases, and in cortical region for those affected by AD. Therefore Lf, and especially the salivary Lf (sLf), could be used as an early AD biomarker. In fact, many studies demonstrate that sLf levels are low in AD patients compared to a healthy control (Reseco et al. 2021).
A specifical focus has been done on bovine lactoferrin (bLf), which showed an effect of regulation of the neuroendocrine system. In particular, bLf is able to down or up-regulate the adrenal corticosteroids by activating the HPA (hypothalamus–pituitary–adrenal) pathway and the opioid nervous system pathway, and by generating nitric oxide (NO). For this reason, bLf can control stress and behaviour thanks to the modulation of hormones and neurotransmitters. Therefore, it can be used in addition to the normal drug-based therapy for emotional disturbances associated with stress (Guzmán-Mejía et al. 2021).
Lactoferrin Against Cancer
As an iron-binding protein, lactoferrin (Lf) has several important activities including the immunomodulatory and anticancer functions (Olszewska et al. 2021). Different types of Lf have these activities: bovine Lf (bLf), human Lf (hLf), recombinant Lf (rhLf), intracellular delta-lactoferrin (delta LF) (Bukowska-Ośko et al. 2022). Lf has two different conformational states that are depending on its iron content: apo-LF (open iron-free form) and holo-LF (closed iron-binding form) (Olszewska et al. 2021). Most of the research concern bLf, that is similar to the hLf: they share a 69% amino acids sequences (Pierce et al. 1991). It has been recently found that Lf can also be used as carrier for chemotherapeutics and, since Lf has the ability to cross the blood–brain barrier, for the treatment of brain tumor. Moreover, administration of Lf does not have significant adverse effects and is highly tolerated (Cutone et al. 2020). This could be related to the fact that Lf is able to either inhibit cell division and movement on cancer cells and to activate cell division and movement on healthy normal cells (Bukowska-Ośko et al. 2022).
Lf can inhibit or prevent cancer cells growth in different ways (Table 2), namely, by activating the adaptive immune response, by decreasing the expression of growth factor protein (i.e. of vascular endothelial growth factor protein in human lung cancer cell line—A549 (Tung et al. 2013)), by increasing the expression of surface receptors on neoplastic cells (facilitating their identification by the immune system), by the inhibition of angiogenesis and the growth of blood vessels to the tumor (Bukowska-Ośko et al. 2022).
The Lf cytotoxicity towards cancer cells is related to cell cycle arrest, cell membrane damage or induction of apoptosis of dangerous cells (Bukowska-Ośko et al. 2022). To be more specific it has been proposed that the anticancer effect is due to two different processes: cell signalling and recognition through the glycans that make up its structure or the interaction of proteoglycan, glycosaminoglycan, and sialic acid with Lf. These mechanisms can also explain the selectivity of Lf against cancer cells and not on healthy cells (Rascón-Cruz et al. 2021). Anyway, by far the most important biological mechanism against tumor development is Lf’s chelating involvement: Lf prevent the increasing of iron in the tissue by trapping this iron on the surface of the tissue. The accumulation of iron in the tissues is dangerous because it leads to oxidative stress (OS) on genetic material, which induces carcinogenesis (Ahmed et al. 2021).
Lf can be used for a lot of different tumor, i.e., colon cancer, breast cancer or lung cancer. It was found, for example, that the oral administration of Lf on rats treated with azoxymethane decreased the occurrence of colon carcinogenesis: 0.2% or 2% respectively decrease of 32.5% and 42.5% (Zhang et al. 2014).
Cell Cycle Arrest and Apoptosis
Lf is different from other chemotherapeutic agents, which do not discriminate between cancer cells and normal healthy cells. In fact, Lf is considered as a selective agent, and it acts as a positive regulator of proliferation in healthy cells, while it has an inhibitory effect against transformed and cancer cells (Cutone et al. 2020).
In general, the anticancer activity of Lf can be split as extracellular effects, intracellular effects and immunostimulation. The cell cycle arrest and apoptosis are related to the intracellular effects (Duarte et al. 2011), because Lf and its derived peptides (i.e., Lactoferricin B, that is a cationic peptides Zhang et al. 2014) are easily internalized by the cells. For example, Lf enters the Jurkat human lymphoblastic T-cells via endocytosis mediated by receptors. The presence of these receptors on the cancer cells surface is demonstrated by many studies (Zhang et al. 2014).
Apoptosis is a mechanism of cell death that protects against tumorigenesis because it brings to death those cells that are going through a cancer transformation. Sometimes it happens that some cells have mutations in their genes that trigger the apoptosis process and keep the cancer cells alive, this leads to the development of a cancerous tumor (Bukowska-Ośko et al. 2022). Lf can both promote and inactivate cell apoptosis (Hou et al. 2014; Kanwar and Kanwar 2013). By regulating the molecules signalling it can activate apoptosis: depending on the tissue/cell type, it can trigger the fas signalling, the mitochondrial-related apoptosis pathway or the V− H+-ATPase-related apoptosis pathway (Bukowska-Ośko et al. 2022). On the opposite, it was found that in osteoblast, osteoclast and neutrophils the Lf inhibits the apoptosis by involving the insuline-like growth factor (IGF) signalling pathway (Hou et al. 2014). This anti-apoptotic effect on neutrophils is useful in the infection site, because this extends the bactericidal function. Lf has this anti-apoptotic effect that depends on its iron saturation status (Bukowska-Ośko et al. 2022).
Immunostimulation and Chemopreventive Potential
Lf has also showed a chemopreventive effect and that is probably due to the fact that Lf has lots of activities that work all at the same time. These activities are the stimulation of the immune response, the modulation of the carcinogen-metabolizing enzymes, the inhibition of angiogenesis and the modulation of the oxidant-antioxidant profile. The key factor between these ones for the chemopreventive effect may be the regulation of the immune function (Zhang et al. 2014). Lf and its derivates regulate both the innate and the adaptive immunity. The function of Lf mainly include the activation of a strong Th1 response and the stimulation of anticancer killing cells (Fischer et al. 2006). It has been showed, for example, that after oral administration of bLf the recruitment of lymphocytes (including primarily CD4+ and CD8+) increase (Wolf et al. 2007). Moreover, it was found that in small intestine the level of interferon-γ, tumor necrosis factor-α (TNF-α), caspase-1, interleukin (IL)-18, and IgM+ and IgA + B cells increase after an oral administration on bLf. (Zhang et al. 2014) Another example concerns the oral administration on bLf in rats that showed an important restriction of VEGF165-mediated angiogenesis (Norrby et al. 2001).
In addition to these activities, Lf has also an important role in the regulation of ion homeostasis of human body: Lf can bind and release some cations (i.e., Zn2+, Fe3+, Cu2+, Mn3+, Ga3+), among which the iron is the most important (Zhang et al. 2014). Iron is a very important cation for human body, and it’s important to always keep the iron homeostasis for the cell growth and enzymes activities. If the iron balance fails, this cation can become toxic because it induces the formation of free radicals. In turn, the radicals produce an oxidative stress that leads to a cellular redox imbalance. This imbalance may leads to oncogenic stimulation (Valko et al. 2006). Lf is important because, by chelating the iron can keep the systemic balance (Zhang et al. 2014).
Lactoferrin in Association with Curcumin for Prostatic Cancer
It was found that curcumin has the ability to activate the apoptotic pathways and reduce cell proliferation. This result was amplified if the curcumin was added with lactoferrin. The combination of these two compounds showed the down regulation of integrin gene expression, the reduction of cells migration, and the up regulation of DR4 and DR5 gene expression. All these mechanisms lead to the reduction of the aggressiveness of prostatic cancer cells. It means that this association of curcumin and lactoferrin may be use as an integration in prostatic cancer therapy (Rascón-Cruz et al. 2021).
Lactoferrin for Gene Expression Modification and DNA Damage Protection
Lf is a very important protein for the balance of the immune system because of its many and variety activities, as decrease of oxidative stress and local inflammation (Bukowska-Ośko, et al. 2022). Lf has a role as protection of DNA damage, and it may be due to both indirect and direct action on human genome. The direct action relates to the DNA binding ability and hydrolyzation of nucleic acids. The indirect action does not concern an interaction with the genetic material but relate to the elimination of DNA damaging factors (such as microbes, hydroxyl radicals and up-regulated immune cells) and their consequences (such as cancer cells). This indirect activity includes iron saturation, cell cycle modulation, scavenging of hydroxyl radicals and antimicrobial and anti-inflammatory function (Bukowska-Ośko et al. 2022).
Immunosuppression-Decrease of Inflammation and Cell Damage
Lf can reduce an inflammatory process and consequently prevents the damage of tissues thanks to its antibacterial ability and thanks to the binding of many pro-inflammatory pathogen associated microbial patterns: Lf avoids the release of pro-inflammatory cytokines and reactive oxygen species, which are response for the tissue damage (Bukowska-Ośko et al. 2022). The immune cells have negative charged groups on their surface and these groups interact with Lf. From this interaction we get a physiological anti-inflammatory reaction due to the activation of signalling pathways (Legrand 2016). In addition, Lf can also regulate cytokine synthesis by modulating gene expression (i.e., by blocking the NF-kB): in particular Lf down regulate the levels of some pro-inflammatory cytokines, such as interleukin 1α, tumor necrosis factor α (TNF-α), interleukin 6 and interleukin 8 (Sabra and Agwa 2020), and it up regulate the production of anti-inflammatory interleukin 10 (IL-19) (Hwang et al. 2007). It is also demonstrated that Lf can activate the monocytes and macrophages in other way: by destroying the complex LPS-binding protein-endotoxin that activates the TLR4 signalling pathway. To destroy the complex, Lf neutralize free LPS (Drago-Serrano et al. 2012).
One of the main processes of immunoregulation of Lf is the control of oxidative stress: Lf alters the production of immunoregulatory mediators. This is fundamental for protection against iron disorders and for the induction of an adaptive immune response (Bukowska-Ośko et al. 2022).
Lactoferrin Controls the Oxidative Stress Induced by ROS
Lactoferrin has a significant role in immune homeostasis and is involved in many hosts’ defence mechanism and response at the level of both organs and cells. In order to keep this homeostasis, Lf controls excessive inflammatory responses through the reduction of oxidative stress at the molecular level (Ogasawara et al. 2014). The human body has his own natural antioxidant defence mechanism that protect from damage. However, sometimes it happens that a lot of reactive oxygen species (ROS) are produced and it occurs the oxidative stress. ROS are reactive compounds containing oxygen that are potentially destructive for the cells. Small perturbation of this homeostasis can be repaired by the cell, which is able, in this case, to trigger the apoptosis. The problem arises when there’s a sever oxidative stress that can lead to tissue necrosis so the death of the cell (Pourzand and Tyrrell 1999). The chemical reaction that leads to the formation of ROS involve free iron radicals, that are toxic compounds either because they produce the ROS and because they damage some components of the cell. To prevent the formation of toxic radicals that cause degenerative diseases it is needed the intervention of antioxidants (Ahmed et al. 2021). bLf is considered as an antioxidant protein thanks to its iron-binding ability (Mulder et al. 2008). Therefore, with this iron sequestration, Lf regulates the physiological homeostasis of ROS and protect cells from oxidative damage (Ahmed et al. 2021).
By activating the expression of catalase, glutathione peroxidase and superoxide dismutase (SOD), Lf can reduce ROS levels (Pan et al. 2021). In a normal situation this three enzymes work together to inactivate radicals: SOD acts first and transform the superoxide radical (·O2−) into hydrogen peroxide (H2O2). Later, catalase and glutathione peroxidase convert H2O2, respectively into water and water plus molecular oxygen (O2) (Kruzel et al. 2017). The situation changes in presence of an excess of free ferric ion (Fe3+): in this case the superoxide radical goes through a non-enzymatic degradation made of two steps. In the first part of the reaction the superoxide radical reacts with the ferric ion (Fe3+) and this generates two products, ferrous ion (Fe2+) and molecular oxygen (O2). At this point the second part of the reaction starts with the ferrous ion (Fe2+) that reacts with hydrogen peroxide (H2O2), this generates three products: a ferric ion (Fe3+), an alcohol and a hydroxyl radical (·OH). This second part is also known as Fenton reaction (Kruzel et al. 2017) (Fig. 4).
The hydroxyl radicals generated from this reaction (Fig. 4a) can be dangerous because they interact with polyunsaturated fatty acids leading to lipid peroxidation, which causes the formation of new radicals called hydroxyalkenals. These radicals are toxic for the cells because they alter the function of some important biomolecules (such as DNA, protein and lipids) (Kruzel et al. 2017) (Fig. 4b). Lactoferrin takes action in the first step of this degradation process: it binds the ferric ion (Fe3+) so the reaction does not even start. In this way, it is avoided the radicals formation and the biomolecular damage (Kruzel et al. 2017). Lactoferrin takes action also in a direct way, that is independent from its iron-binging ability (Fig. 4c). Lf has the ability to protect DNA in vitro by the scavenging of ·OH radicals. Maybe the mechanism of this protection is based on a specific structure in Lf molecules that interacts with oligonucleotides, and this result as a DNA protection to direct oxidative injury (Ogasawara et al. 2014).
Lactoferrin for Obesity
It was recently found that Lf has a significant effect on decreasing weight gain. Hassan et al. has administered supplemented lactoferrin with stirred yogurt in obese animals, and the result was not only the decreasing of weight gain, but also the decreasing of serum glucose concentration and the reduction of lipid levels. It was possible because of the ability of Lf to restore the cellular antioxidant function. Moreover, the same study showed that the administration of two different dose of Lf supplemented with stirred yogurt (50 and 100 mg/kg) reduced the complication of pancreas injury thanks to the normalization of pancreatic enzymes (Hassan et al. 2022).
Another work from Zapata et al. showed some positive effects of lactoferrin compared to a control. First, Lf produced weight and fat loss. Secondly, it was observed a reduction in glucose excursions after Lf administration: this is important because it leads to the glycemic improvement. Third, Lf reduces also the hepatic lipidosis: this is possible thanks to Lf’s ability to induce an increase of β-oxidation transcripts in the liver and Lf’s ability to reduces the lipogenic regulation. Finally, Lf showed and increasing in plasma levels of two different compounds: anorexigenic hormones and a specific cecal bacteria population, which is able to improve glycemia and energy homeostasis (Zapata et al. 2017).
Ono et al. studied the effects of oral administration of Lf tablets for 8 weeks. The results were an important decrease of visceral fat accumulation and reduction of hip circumference, BMI, body weight and total fat area. No particular adverse events concerning safety were encountered (Ono et al. 2010).
From a study on obese subjects it was possible to understand that genes related to lactoferrin’s receptor may be related to a prevalence of metabolically unhealthy or metabolically healthy phenotypes (Jamka et al. 2020).
One of the most recent study about lactoferrin reported its effect also in combination with hypoxia. In fact, Lf alleviate obesity both when it’s alone and when combined. Alone Lf reduce body weight, improve the gut flora composition, controls food intake, reduce lipid deposition thanks to lipid metabolism (fat synthesis and transport) and bile acid metabolism regulation. When combined with hypoxia and administered in high fat feeding mice, Lf gave even better results, improving not only the body weight, but also the blood quality and the pathological indices. Moreover, this combination has protective effects such as alleviating the hepatic steatosis and improving of cholesterol synthesis, transport and clearance (Wu et al. 2023).
It has been showed that circulating levels of Lf can be related to overweight/obese Latino youth. Kim et al. (Kim et al. 2015) identified that Lf, such as a lot of other genes, are express differently in overweight/obese youth. In particular, Lf is the most strongly up-regulated one between these genes. High level of circulating Lf were, therefore, associated to high BMI, fat mass, hip and waist circumferences. These results are the opposite of the adult situation (Kim et al. 2015).
Lf Formulations in the Food and Biomedical Fields
Lf is isolated from colostrum and unpasteurized whey using chromatographic techniques and separative membranes. In fact, because Lf is sensitive to heat above certain temperatures, pasteurized milk is not suitable for Lf purification. Sterilization processes such as UHT involve exposing the protein to temperatures between 130 and 150 °C for a few seconds, and this appears to damage the structure of the protein and so also some functions such as antibacterial (Artym et al. 2021). The most widely used chromatographic technique is cation exchange, in fact Lf is a positively charged protein in serum and can therefore be retained by negatively charged resins before being washed out with highly saline solutions. After which Lf is desalted and concentrated by membrane filtration and then freeze-dried or spray-dried to powder with a high degree of purity (more than 90%). The most suitable pH values for the processes described are a pH 5 for adsorption and pH 10 for desorption. Other combined techniques that purified LF from sweet whey using MF and UF techniques followed by expanded-bed chromatography based on cationic exchange have also been tested in recent studies, obtaining excellent results, a purity of 92.7% and recovery of 87.0%. UF electrodialysis techniques have also been tested, but in this case the main problem concerns the difficulty in separating Lf from other proteins that migrate together (Lin et al. 2021). Once pure salmon pink powder is obtained (due to the presence of Fe), the administration of Lf may involve different formulations that have been tested and are still under study, involving routes of administration such as intravenous, intravaginal, intranasal, sublingual, and oral, as well as topical on wounds (Artym et al. 2021).
Although its industrial production has not yet started, an alternative to extracting it from milk is the production of recombinant human lactoferrin (rhLf), i.e., produced through genetic engineering techniques. RhLf can be obtained introducing the Lf’s gene into a host cell like a mammalian one or a bacterial one. The cell will produce Lf that can be purified and used as a supplement as well, since it maintains the properties of the physiological one (Ashraf 2023).
Nanoparticles for Eye Delivery
Lf and its formulations as nanoparticles are applied to various ocular pathologies of the cornea and retina such as retinis pigmantosa (RP), retinoblastoma, age-related macular degeneration (AMD), uveitis, and keratoconus. The retina is particularly at risk of oxidative stress, since photoreceptors are constantly exposed to light that induces ROS accumulation in the retinal pigment epithelium (RPE). Lf due to its properties it has been applied also for the treatment of numerous eye diseases such as viral or bacterial infections, and conjunctivitis. Nanoparticles of LF have been formulated for topical application to treat these inflammatory diseases of the retina and cornea (Kell et al. 2020; Singh et al. 2022).
LF NPs in the conducted study were prepared by the sol-oil preparation method by dissolving LF powder (0.2%) in a phosphate buffered saline under stirring for one hour at 25 °C. After the hour had elapsed, it was then found as the best condition for forming the nanoparticles, that of bringing the solution to a pH value of 7 for 20 min at 75 °C. Nanoparticles were then characterized and analyzed. They were then applied in vitro and in vivo, demonstrating the great potential of this protein but also of the delivery system that is able to increase permeability across the eye barriers and allow prolonged release over time (Singh et al. 2022).
In another study, moreover, in order to use Lf in the treatment of the keratoconus disease, nanostructured lipid carriers (NLCs) have been formulated via by a double emulsion/solvent evaporation method. Thanks to this new lipid nanosystem, a high loading id capacity can be achieved and at the same time a control in drug release. In addition, this formulation was found to be nontoxic and with mucoadhesive properties. Keratoconus, in particular, is a degenerative ecstatic disease of the cornea that leads to bilateral visual impairment that is destined to worsen over time and for which there are no effective, non-invasive treatments. Although the etiology of the disease is still not entirely clear, some elements related to the disease itself have been identified, for example, an increase in inflammation factors such as cytokines and cell adhesion glycoproteins. Indeed, there is overexpression of TNF-alpha, IL-6 and MMP-9 as key factors in disease progression. Lf has also been shown to stimulate the healing of wounds to the cornea and to play a role in the treatment of keratoconus. Lf levels were lower in keratoconus subjects; in addition, Lf influences the immune response and interacting with intra- and extracellular pathways involving TLRs, regulates levels of cytokines and other mediators of inflammation (Varela-Fernández et al. 2022).
Also in the field of ocular therapy, a thermosensitive hydrogel consisting of PLGA-PEG-PEI nanoparticles containing Amphotericin-B and Lf was formulated for the treatment of fungal infections in the eye. Lf plays the role of anti-inflammatory and prevents dry eyes, assisting Amphotericin-B which is antifungal against Candida albicans, Fusarium, and Aspergillus flavus in the treatment of symptoms (Elhabal et al. 2023).
Intranasal Delivery
Dopamine with co-modified borneol and lactoferrin nanoparticles (Lf-BNPs) by intranasal delivery has been formulated for the treatment of Parkinson's disease. The NPs were formulated using the double emulsion solvent evaporation method. Encapsulating dopamine in such a delivery system made it possible on the one hand to reduce side effects and on the other hand to achieve an effective delivery to the brain. In vitro tests performed showed low toxicity on the tested cell lines and the presence of Lf increases the cellular uptake of NPs. In vivo intranasal administration of dopamine Lf-BNPs effectively alleviates lesions to the striatum in rats. The data obtained therefore suggest that this intranasal administration may be an effective delivery system in the treatment of PD. Lf is reconfirmed to be an excellent ligand for drug delivery to striatal tissue, in fact receptors for Lf are overexpressed on the apical surface of the respiratory epithelium but also in capillaries and neui neurons in neurodegenerative diseases such as PD and Alzheimer’s (Tang et al. 2019).
Another recent study in 2023 tested the effectiveness of self-assembled curcumin-lactoferrin nanoparticles for nose-to-brain delivery. Curcumin is a natural polyphenol with anti-inflammatory and antioxidant properties that can be exploited to achieve neuroprotection against CNS neurodegenerative diseases. CUR’s effects are synergistic with respect to those of Lf, which also in the formulation plays the role of nanocarrier and targeting ligand in the delivery of CUR-LF NPs. The NPs were obtained by binding the hydrophobic region of LF to CUR without the need to introduce a cross-linking agent. The resulting NPs were found to be effectively internalized by PC12 cells, effective in reducing oxidative stress and apoptosis. Therefore, they showed promising results in achieving neuroprotection (Elhabal et al. 2023).
Moreover, intranasal administration of human Lf in a mouse model suffering from allergic rhinitis showed a reduction in symptoms and inflammation. in addition, a reduction in inflammation was also obtained in studies performed on cell cultures of mouse bronchial epithelium tissue (Presti et al. 2021).
Dermal Delivery
Regarding a topical application, a gelatin hydrogel with bio-nanosilver functionalized with lactoferrin (Ag-LTF) has been formulated, thus having dual antimicrobial action for use in the treatment of infected and chronic wounds. Formulation has been found to be nontoxic and safe and to prevent biofilm formation, improving healing of infected wounds (Abdalla et al. 2021).
Topical Lf also inhibits allergen-induced and Langerhans cell migration; in another double-blind study conducted using Lf in combination with an Ig-rich serum fraction benefits were obtained in the treatment of atopic dermatitis (Presti et al. 2021).
Oral Delivery
Oral preparations of Lf have also been found to be safe and effective as well as practical. In fact, Lf derived from cow's milk has been approved by the FDA and EFSA for years and recognized as Generally Recognized as Safe (GRAS), so it can be used as a supplement in the diet. Lf administered orally (e.g., in capsules) it is able to resist enzymatic degradation especially when taken during meals where gastric acidity is mitigated by the presence of food, or when taken together with calcium-rich foods that stabilize its structure. It has been shown that external coating lactoferrin has better uptake in the small intestine and that enteric coating protects against enzymatic degradation in the stomach and allows absorption and transit to the systemic circulation (Ashraf 2023).
Lf supplementation leads to changes in the gut microbiota, increasing the proliferation of beneficial bacteria and the decrease of harmful strains, increases the innate and adaptive immune response and is able to increase the strength and weight of bone tissue (Ashraf 2023).
Orally administered Lf knows how to reach the gut where it interacts with the microbiota, epithelium, and gut associated lymphoid tissue (GALT). This tissue is activated by Lf and leads to a release of cytokines and immune cells that through the circulatory stream can reach membranes and mucous membranes systemically, increasing immunity. In this way Lf protects mucous membranes such as the ocular, gastrointestinal, urinary, and reproductive tract from pathogen penetration and excessive inflammatory responses (Artym et al. 2021). In fact one of the most investigated and actual uses is the use of Lf as a supplement in infant formulas. Studies conducted have shown that infants fed Lf-enriched formulas have fewer episodes of diarrhea and respiratory infections than the control group. In another study, children fed a formula enriched in Lf and bovine milk fat globule membranes showed significant improvement in cognitive, language and motor skills at 12 months of age. Lf is in fact currently present in some infant formulas since EFSA concluded that Lf is safe as a novel food ingredient when added to formulations in amounts of 1000 mg/L for up to 1.1 g/day (Ashraf 2023). Actually, Lf is used not only in infant formula but also in yogurt, skimmed milk, and beverages (Einerhand et al. 2022).
In addition, Lf administered orally to mice showed an increase in the expression of many genes such as those coding for certain cytokines and cellular receptors, in a manner comparable to the effect of Lf administered intravenously. Moreover, oral Lf stimulates myelopoiesis and the regulation of IL-6 and TNF-a production in the blood of healthy volunteers and when administered to patients before surgery it has been shown to reduce the decrease in postoperative immune response. Other interesting results were obtained by administering Lf orally to pregnant women along with iron and vitamins, the administration led to improve hematological parameters, prolonged time of pregnancy (preventing preterms birth) and to an increase in new-born weight (Artym et al. 2021). As already discussed before, Lf orally administered as well as intranasal delivery of Lf can also lead to the improvement of COVID-19 symptoms.
Some studies have tested the administration of Lf for the treatment of iron-deficiency anaemia, which mainly affects poor or developing countries and impairs the proper development of the immune system and cognitive development. The main problem, however, is that the oral administration of spelt often leads to it passing through the intestine without being absorbed and leading to side effects such as changes in the intestinal microbiota, inflammation and diarrhea. A study in children in Kenya showed that co-administration with apo-Lf and probiotics significantly improved iron absorption and reduced side effects (Zimmermann 2020).
In a recent study, moreover, Lf has been used in oral delivery for the treatment of colon cancer. In fact, Lf nanoparticles, which are an excellent delivery system in cancer therapy, were microencapsulated into calcium-crosslinked microparticles using polysaccharide-protein hybrid copolymers to protect them from GIT tract degradation and to reduce the burst effect. This nanoparticle microparticle delivery system (NIMDs) containing docetaxel (DTX) and atorvastatin (ATR) as active ingredients contained into Lf backbone showed good ability to protect drugs from degradation, deliver them to the desired intestinal tract, and increase therapeutic effect by suppressing p-AKT, p-ERK1/2, and NF-κB (Elmorshedy et al. 2023).
Another recent study demonstrated the role of oral Lf in preventing preterm delivery due to ascending genital tract infections. Lf's mechanism of action in counteracting vaginal infections has not been fully elucidated but it appears that Lf knows how to increase IgA production in the GI tract and activate immune cells leading to improved GI and vaginal tract. It thus protects cervical tissue from inflammation and reduces episodes of PTB and bacterial vaginitis (Otsuki et al. 2023).
Intravaginal Delivery
As with oral administration of Lf, even when administered in situ vaginally, this protein showed action in preventing preterm birth when administered to women with bacterial vaginitis in the first trimester. In the study, the incidence of PTB at less than 37 weeks of gestations in women who took vaginal tablets with 300 mg of Lf daily compared with those who did not take it was compared. It was observed that in treated women the rate of PTB < 37 weeks was significantly decreased (Miranda et al. 2021).
In addition, another study also looked at measuring the levels of cytokines, chemokines and growth factors present in amniotic fluid after administration of vaginal Lf in three different groups. The first group consisted of untreated women, the second group of women who took Lf 300 mg 4 h before amniocentesis, and the third group of patients treated with the same dose 12 h before amniocentesis. The study showed that Lf can down-regulate 17 pro-inflammatory cytokines and up-regulate five anti-inflammatory mediators showing a protective role against inflammatory complications in pregnancy (Maritati et al. 2017).
Conclusions
Increasing life expectancy by decreasing the risk of age-related diseases, such as neurodegenerative diseases and cancers, is a continuous aim of scientific and clinical research. One such compound being studied for these purposes is lactoferrin, a major iron-binding protein found in milk. The protein, discovered as early as 1960, still represents a current tool to address some of the most urgent and complicated challenges such as the increase in diseases caused by oxidative stress, drug resistance by some bacteria and fungi, and not least viral pandemics. This literature review demonstrated that Lf may play important roles as antibacterial, antiviral, anticancer, antioxidant and anti-obesity agent, among others. All these properties derive mainly from Lf's ability to bind iron, thus maintaining homeostasis of this ion levels in the body with all that follows. In fact, Lf binds iron by sequestering it from microorganisms that need it for survival and replication, subtracting it from the Fenton reaction that produces ROS in cells, thus decreasing oxidative stress, damage to cellular structures, and aging. Lf can also interact with different receptors and cellular structures due to its three-dimensional structure, stimulating them in some cases (e.g., leading to a regulation of the immune response) and inhibiting them in others (by masking the ACEII receptor on cells, it prevents e.g., the infection of Sars-Cov-2 in the host cell). Thanks to these transversal properties, Lf results in a wide variety of applications with action at the level of various organs and useful for the resolution of pathologies typical of all ages of life (from reducing the number of preterm births to typical problems of old age such as neurodegenerative ones). In addition, since it is an endogenous and safe molecule, declared as Generally Recognized as Safe (GRAS) by the FDA and EFSA agencies, it is already in use as a prebiotic and adjuvant in mucous membranes such as the oral mucosa and in food preparations for children. This scientific topic still deserves further studies to characterize this glycoprotein, as well as coupling drugs to Lf.
References
Abdelhamid M et al (2020) Dietary lactoferrin supplementation prevents memory impairment and reduces amyloid-β generation in J20 mice. J Alzheimers Dis 74(1):245–259
Aguilar-Diaz H et al (2017) Parasiticidal effect of synthetic bovine lactoferrin peptides on the enteric parasite Giardia intestinalis. Biochem Cell Biol 95(1):82–90
Ahlawat S, Sharma KK (2020) Immunological co-ordination between gut and lungs in SARS-CoV-2 infection. Virus Res 286:198103
Ahmed KA et al (2021) Lactoferrin: potential functions, pharmacological insights, and therapeutic promises. J Adv Biotechnol Exp Ther 4(2):223
Algahtani FD et al (2021) The prospect of lactoferrin use as adjunctive agent in management of SARS-CoV-2 patients: a randomized pilot study. Medicina (kaunas) 57(8):842
Ali AS et al (2021) Lactoferrin reduces the risk of respiratory tract infections: a meta-analysis of randomized controlled trials. Clin Nutr ESPEN 45:26–32
Al-Mogbel MS et al (2021) Effect of synergistic action of bovine lactoferrin with antibiotics on drug resistant bacterial pathogens. Medicina 57(4):343
Ammendolia MG, Marchetti M, Superti F (2007) Bovine lactoferrin prevents the entry and intercellular spread of herpes simplex virus type 1 in Green Monkey Kidney cells. Antivir Res 76(3):252–262
Andersen JH, Jenssen H, Gutteberg TJ (2003) Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antivir Res 58(3):209–215
Andersen JH et al (2004) Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J Med Virol 74(2):262–271
Andreu S et al (2023) Liposomal lactoferrin exerts antiviral activity against HCoV-229E and SARS-CoV-2 pseudoviruses in vitro. Viruses 15(4):972
Arnold RR et al (1982) Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect Immun 35(3):792–799
Arredondo-Beltrán IG et al (2023) Antitumor activity of bovine lactoferrin and its derived peptides against HepG2 liver cancer cells and Jurkat leukemia cells. Biometals 36:639
Artym J, Zimecki M (2013) Milk-derived proteins and peptides in clinical trials. Postepy Hig Med Dosw 67:800–816
Artym J et al (2018) Immunomodulatory properties of human recombinant lactoferrin in mice: implications for therapeutic use in humans. Adv Clin Exp Med 27(3):391–399
Artym J, Zimecki M, Kruzel ML (2021) Lactoferrin for prevention and treatment of anemia and inflammation in pregnant women: a comprehensive review. Biomedicines 9(8):898
Ashraf MF et al (2023) Nutraceutical and health-promoting potential of lactoferrin, an iron-binding protein in human and animal: current knowledge. Biol Trace Elem Res 2013:1–17
Avalos-Gómez C et al (2022) Lactoferrin: an effective weapon in the battle against bacterial infections. Curr Pharm Des 28(40):3243–3260
Baker EN, Baker HM (2005) Lactoferrin. Cell Mol Life Sci 62(22):2531
Baker EN, Baker HM (2009) A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 91(1):3–10
Baker HM, Baker EN (2012) A structural perspective on lactoferrin function. Biochem Cell Biol 90(3):320–328
Baker HM et al (2000) Metal substitution in transferrins: specific binding of cerium(IV) revealed by the crystal structure of cerium-substituted human lactoferrin. J Biol Inorg Chem 5(6):692–698
Bennett RM, Bagby GC, Davis J (1981) Calcium-dependent polymerization of lactoferrin. Biochem Biophys Res Commun 101(1):88–95
Berkhout B et al (2002) Characterization of the anti-HIV effects of native lactoferrin and other milk proteins and protein-derived peptides. Antivir Res 55(2):341–355
Berlov M et al (2007) Lactoferrin from canine neutrophils: isolation and physicochemical and antimicrobial properties. Biochem Mosc 72:445–451
Berlutti F et al (2011) Antiviral properties of lactoferrin–a natural immunity molecule. Molecules 16(8):6992–7018
Bolat E et al (2022) Lactoferrin for COVID-19 prevention, treatment, and recovery. Front Nutr 9:992733
Bukowska-Ośko I, Popiel M, Kowalczyk P (2021) The immunological role of the placenta in SARS-CoV-2 infection: viral transmission, immune regulation, and lactoferrin activity. Int J Mol Sci 22(11):5799
Bukowska-Ośko I et al (2022) Lactoferrin as a human genome “Guardian”: an overall point of view. Int J Mol Sci 23(9):5248
Campione E et al (2021) Lactoferrin against SARS-CoV-2: in vitro and in silico evidences. Front Pharmacol 12:666600
Chang R, Ng TB, Sun WZ (2020) Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int J Antimicrob Agents 56(3):106118
Chapple DS et al (2004) Structure and association of human lactoferrin peptides with Escherichia coli lipopolysaccharide. Antimicrob Agents Chemother 48(6):2190–2198
Chen K et al (2016) Effect of bovine lactoferrin from iron-fortified formulas on diarrhea and respiratory tract infections of weaned infants in a randomized controlled trial. Nutrition 32(2):222–227
Cutone A et al (2020) Lactoferrin’s anti-cancer properties: safety, selectivity, and wide range of action. Biomolecules 10(3):456
Dai Y-J et al (2020) A profiling analysis on the receptor ACE2 expression reveals the potential risk of different type of cancers vulnerable to SARS-CoV-2 infection. Ann Transl Med 8(7):481
de Araújo AN, Giugliano LG (2001) Lactoferrin and free secretory component of human milk inhibit the adhesion of enteropathogenic Escherichia coli to HeLa cells. BMC Microbiol 1:25
De Pasquale V et al (2021) Heparan sulfate proteoglycans in viral infection and treatment: a special focus on SARS-CoV-2. Int J Mol Sci 22(12):6574
Drago-Serrano ME et al (2012) Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int Immunopharmacol 12(1):1–9
Drobni P, Näslund J, Evander M (2004) Lactoferrin inhibits human papillomavirus binding and uptake in vitro. Antivir Res 64(1):63–68
Duarte D et al (2011) The effect of bovine milk lactoferrin on human breast cancer cell lines. J Dairy Sci 94(1):66–76
Dzitko K et al (2007) Toxoplasma gondii: inhibition of the intracellular growth by human lactoferrin. Pol J Microbiol 56(1):25
Einerhand AWC et al (2022) Can lactoferrin, a natural mammalian milk protein, assist in the battle against COVID-19? Nutrients 14(24):5274
Elhabal SF et al (2023) Development of thermosensitive hydrogel of Amphotericin-B and Lactoferrin combination-loaded PLGA-PEG-PEI nanoparticles for potential eradication of ocular fungal infections: In-vitro, ex-vivo and in-vivo studies. Int J Pharm X 5:100174
Elmorshedy YM et al (2023) Engineered microencapsulated lactoferrin nanoconjugates for oral targeted treatment of colon cancer. Biomacromol 24(5):2149–2163
Elzoghby AO et al (2020) Lactoferrin, a multi-functional glycoprotein: active therapeutic, drug nanocarrier & targeting ligand. Biomaterials 263:120355
Enjuanes L et al (2016) Molecular basis of coronavirus virulence and vaccine development. Adv Virus Res 96:245–286
Fais R et al (2022) Synergistic activity of the human lactoferricin-derived peptide hLF1-11 in combination with caspofungin against candida species. Microbiol Spectr 10(4):e0124022
Farnaud S et al (2004a) Variation in antimicrobial activity of lactoferricin-derived peptides explained by structure modelling. FEMS Microbiol Lett 238(1):221–226
Farnaud S et al (2004b) Interactions of lactoferricin-derived peptides with LPS and antimicrobial activity. FEMS Microbiol Lett 233(2):193–199
Fischer R et al (2006) Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem Cell Biol 84(3):303–311
Furlund C et al (2013) Identification of lactoferrin peptides generated by digestion with human gastrointestinal enzymes. J Dairy Sci 96(1):75–88
González-Chávez SA, Arévalo-Gallegos S, Rascón-Cruz Q (2009) Lactoferrin: structure, function and applications. Int J Antimicrob Agents 33(4):301.e1-301.e8
Gruden Š, Poklar-Ulrih N (2021) Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrin-derived peptides. Int J Mol Sci 22(20):11264
Guzmán-Mejía F et al (2021) Bovine lactoferrin as a modulator of neuroendocrine components of stress. Curr Mol Pharmacol 14(6):1037–1045
Hao L et al (2019) Lactoferrin: major physiological functions and applications. Curr Protein Pept Sci 20(2):139–144
Hara K et al (2002) Lactoferrin inhibits hepatitis B virus infection in cultured human hepatocytes. Hepatol Res 24(3):228–235
Hassan MA, Abedelmaksoud TG, Abd El-Maksoud AA (2022) Effects of lactoferrin supplemented with fermented milk on obesity-associated pancreatic damage in rats. Life (basel) 12(12):201
He ST et al (2023) Bovine lactoferrin inhibits SARS-CoV-2 and SARS-CoV-1 by targeting the RdRp complex and alleviates viral infection in the hamster model. J Med Virol 95(1):e28281
Ho Y-H, Sung T-C, Chen C-S (2012) Lactoferricin B inhibits the phosphorylation of the two-component system response regulators BasR and CreB. Mol Cell Proteomics 11(4):M111.014720
Hou JM et al (2014) Lactoferrin inhibits apoptosis through insulin-like growth factor I in primary rat osteoblasts. Acta Pharmacol Sin 35(4):523–530
Hu Y et al (2021) The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor. Emerg Microbes Infect 10(1):317–330
Hwang SA et al (2007) Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med Microbiol Immunol 196(3):171–180
Ikoma-Seki K et al (2015) Role of LRP1 and ERK and cAMP signaling pathways in lactoferrin-induced lipolysis in mature rat adipocytes. PLoS ONE 10(10):e0141378
Jaber BA et al (2022) Clinical trials on alternative medicines for COVID-19. Stud Health Technol Inform 295:366–369
Jamka M et al (2020) Metabolic health in obese subjects: is there a link to lactoferrin and lactoferrin receptor-related gene polymorphisms? Nutrients 12(9):2843
Jiang R, Lönnerdal B (2017) Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways. Biochem Cell Biol 95(1):99–109
Johansson C et al (2007) Adenoviruses use lactoferrin as a bridge for CAR-independent binding to and infection of epithelial cells. J Virol 81(2):954–963
Jose-Abrego A et al (2022) Anti-hepatitis B virus activity of food nutrients and potential mechanisms of action. Ann Hepatol. https://doi.org/10.1016/j.aohep.2022.100766
Kaczyńska K et al (2023) Potential of lactoferrin in the treatment of lung diseases. Pharmaceuticals 16(2):192
Kalmar JR, Arnold RR (1988) Killing of Actinobacillus actinomycetemcomitans by human lactoferrin. Infect Immun 56(10):2552–2557
Kanwar RK, Kanwar JR (2013) Immunomodulatory lactoferrin in the regulation of apoptosis modulatory proteins in cancer. Protein Pept Lett 20(4):450–458
Karav S (2018) Selective deglycosylation of lactoferrin to understand glycans’ contribution to antimicrobial activity of lactoferrin. Cell Mol Biol (noisy-Le-Grand) 64(9):52–57
Karav S et al (2017) Studying lactoferrin N-glycosylation. Int J Mol Sci 18(4):870
Kautto L et al (2016) Glycan involvement in the adhesion of Pseudomonas aeruginosa to tears. Exp Eye Res 145:278–288
Kawasaki Y et al (2000) Inhibitory effects of bovine lactoferrin on the adherence of enterotoxigenic Escherichia coli to host cells. Biosci Biotechnol Biochem 64(2):348–354
Kell DB, Heyden EL, Pretorius E (2020) The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front Immunol 11:1221
Kim JY et al (2015) Gene expression profiling and association of circulating lactoferrin level with obesity-related phenotypes in Latino youth. Pediatr Obes 10(5):338–344
Kopaeva Y et al (2019) Transport of human lactoferrin into mouse brain: administration routes and distribution. Bull Exp Biol Med 167(4):561–567
Kowalczyk P et al (2022) The lactoferrin phenomenon-a miracle molecule. Molecules 27(9):2941
Krupińska AM, Bogucki Z (2021) Clinical aspects of the use of lactoferrin in dentistry. J Oral Biosci 63(2):129–133
Kruzel ML, Zimecki M, Actor JK (2017) Lactoferrin in a context of inflammation-induced pathology. Front Immunol 8:1438
Legrand D (2016) Overview of lactoferrin as a natural immune modulator. J Pediatr 173(Suppl):S10–S15
Lepanto MS et al (2019) Lactoferrin in aseptic and septic inflammation. Molecules 24(7):1323
Li YQ, Guo C (2021) A review on lactoferrin and central nervous system diseases. Cells 10(7):1810
Li Y et al (2021) Lactoferrin is a potential activator of the vitamin D receptor in its regulation of osteogenic activities in C57BL/6J mice and MC3T3-E1 cells. J Nutr 151(8):2105–2113
Li B et al (2022) The effect of lactoferrin in aging: role and potential. Food Funct 13(2):501–513
Liao H et al (2019) Enhanced antifungal activity of bovine lactoferrin-producing probiotic Lactobacillus casei in the murine model of vulvovaginal candidiasis. BMC Microbiol 19(1):7
Lin T et al (2021) Bioactives in bovine milk: chemistry, technology, and applications. Nutr Rev 79(Suppl 2):48–69
Liu Y et al (2011) Comparative antimicrobial activity and mechanism of action of bovine lactoferricin-derived synthetic peptides. Biometals 24(6):1069–1078
Liu H et al (2020) Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease in mice. J Neurochem 152(3):397–415
Lizzi A et al (2016) Bovine lactoferrin and its tryptic peptides: antibacterial activity against different species. Appl Biochem Microbiol 52:435–440
López-Otín C et al (2013) The hallmarks of aging. Cell 153(6):1194–1217
Mahendra B, Kavitha R (2021) A review on COVID19: evolution, structure of corona virus and comparison of COVID19 with common FLU. Shanlax Int J Arts Sci Humanit 8:54–59
Manzoni P (2019) Clinical studies of lactoferrin in neonates and infants: an update. Breastfeed Med 14(S1):S-25-S−27
Manzoni P et al (2012) Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics 129(1):116–123
Maritati M et al (2017) Influence of vaginal lactoferrin administration on amniotic fluid cytokines and its role against inflammatory complications of pregnancy. J Inflamm (lond) 14:5
Masson P, Heremans J (1971) Lactoferrin in milk from different species. Comp Biochem Physiol 1:119–129
Masson P, Heremans J, Dive C (1966) An iron-binding protein common to many external secretions. Clin Chim Acta 14(6):735–739
Matino E et al (2023) Effect of lactoferrin on clinical outcomes of hospitalized patients with COVID-19: the LAC randomized clinical trial. Nutrients 15(5):1285
Mika JT et al (2011) Structural basis for the enhanced activity of cyclic antimicrobial peptides: the case of BPC194. Biochim Biophys Acta 1808(9):2197–2205
Milewska A et al (2014) Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J Virol 88(22):13221–13230
Miotto M et al (2021a) Molecular mechanisms behind anti SARS-CoV-2 action of lactoferrin. Front Mol Biosci 8:607443
Mirabelli C et al (2021) Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19. Proc Natl Acad Sci 118(36):e2105815118
Miranda M et al (2021) Vaginal lactoferrin in prevention of preterm birth in women with bacterial vaginosis. J Matern Fetal Neonatal Med 34(22):3704–3708
Mistry N et al (2007) The anti-papillomavirus activity of human and bovine lactoferricin. Antivir Res 75(3):258–265
Moore SA et al (1997) Three-dimensional structure of diferric bovine lactoferrin at 2.8 A resolution. J Mol Biol 274(2):222–236
Mrityunjaya M et al (2020) Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol 11:2337
Mulder AM et al (2008) Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr Res 28(9):583–589
Navarro R et al (2023) Bovine lactoferrin for the prevention of COVID-19 infection in health care personnel: a double-blinded randomized clinical trial (LF-COVID). Biometals 36(3):463–472
Nikaido H (2003) Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67(4):593–656
Nikaido H, Vaara M (1985) Molecular basis of bacterial outer membrane permeability. Microbiol Rev 49(1):1–32
Norrby K et al (2001) Orally administered bovine lactoferrin systemically inhibits VEGF(165)-mediated angiogenesis in the rat. Int J Cancer 91(2):236–240
Oda H et al (2021) Antiviral effects of bovine lactoferrin on human norovirus. Biochem Cell Biol 99(1):166–172
Ogasawara Y et al (2014) Lactoferrin directly scavenges hydroxyl radicals and undergoes oxidative self-degradation: a possible role in protection against oxidative DNA damage. Int J Mol Sci 15(1):1003–1013
Olszewska P, Pazdrak B, Kruzel ML (2021) A novel human recombinant lactoferrin inhibits lung adenocarcinoma cell growth and migration with no cytotoxic effect on normal human epithelial cells. Arch Immunol Ther Exp 69(1):33
Ono T et al (2010) Potent anti-obesity effect of enteric-coated lactoferrin: decrease in visceral fat accumulation in Japanese men and women with abdominal obesity after 8-week administration of enteric-coated lactoferrin tablets. Br J Nutr 104(11):1688–1695
Orsi N (2004) The antimicrobial activity of lactoferrin: current status and perspectives. Biometals 17(3):189–196
Otsuki K et al (2023) Review, role of lactoferrin in preventing preterm delivery. Biometals 36(3):521–530
Pan Y et al (2021) Evaluation of the anti-inflammatory and anti-oxidative effects of therapeutic human lactoferrin fragments. Front Bioeng Biotechnol 9:779018
Pawar S, Markowitz K, Velliyagounder K (2022) Effect of human lactoferrin on Candida albicans infection and host response interactions in experimental oral candidiasis in mice. Arch Oral Biol 137:105399
Piacentini R et al (2022) Lactoferrin inhibition of the complex formation between ACE2 receptor and SARS CoV-2 recognition binding domain. Int J Mol Sci 23(10):5436
Pierce A et al (1991) Molecular cloning and sequence analysis of bovine lactotransferrin. Eur J Biochem 196(1):177–184
Pietrantoni A et al (2003) Bovine lactoferrin inhibits adenovirus infection by interacting with viral structural polypeptides. Antimicrob Agents Chemother 47(8):2688–2691
Pietrantoni A et al (2010) Bovine lactoferrin inhibits influenza A virus induced programmed cell death in vitro. Biometals 23:465–475
Pourzand C, Tyrrell RM (1999) Apoptosis, the role of oxidative stress and the example of solar UV radiation. Photochem Photobiol 70(4):380–390
Presti S et al (2021) Lactoferrin: cytokine modulation and application in clinical practice. J Clin Med 10(23):5482
Ramírez-Sánchez DA et al (2021) Bovine lactoferrin and lactoferrin peptides affect endometrial and cervical cancer cell lines. Biochem Cell Biol 99(1):149–158
Rascón-Cruz Q et al (2021) Lactoferrin: a glycoprotein involved in immunomodulation, anticancer, and antimicrobial processes. Molecules 26(1):205
Reseco L et al (2021) Salivary lactoferrin is associated with cortical amyloid-beta load, cortical integrity, and memory in aging. Alzheimer’s Res Ther 13(1):150
Rosa L et al (2017) Lactoferrin: a natural glycoprotein involved in iron and inflammatory homeostasis. Int J Mol Sci 18(9):1985
Russo R et al (2019) Randomised clinical trial in women with recurrent vulvovaginal candidiasis: efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses 62(4):328–335
Sabra S, Agwa MM (2020) Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int J Biol Macromol 164:1046–1060
Saito H et al (1991) Potent bactericidal activity of bovine lactoferrin hydrolysate produced by heat treatment at acidic pH. J Dairy Sci 74(11):3724–3730
Serrano G et al (2020) Liposomal lactoferrin effect in preventing SARS-CoV-2 binding in HACAT keratinocytes. Int J Res Health Sci 8:16–21
Shereen MA et al (2020) COVID-19 infection: emergence, transmission, and characteristics of human coronaviruses. J Adv Res 24:91–98
Singh J et al (2022) Lactoferrin and its nano-formulations in rare eye diseases. Indian J Ophthalmol 70(7):2328–2334
Siqueiros-Cendón T et al (2014a) Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin 35(5):557–566
Spagnuolo PA, Hoffman-Goetz L (2008) Dietary lactoferrin does not prevent dextran sulfate sodium induced murine intestinal lymphocyte death. Exp Biol Med (maywood) 233(9):1099–1108
Suleman Ismail Abdalla S et al (2021) Gelatin hydrogels loaded with lactoferrin-functionalized bio-nanosilver as a potential antibacterial and anti-biofilm dressing for infected wounds: synthesis, characterization, and deciphering of cytotoxicity. Mol Pharm 18(5):1956–1969
Superti F et al (2019) Bovine lactoferrin prevents influenza A virus infection by interfering with the fusogenic function of viral hemagglutinin. Viruses 11(1):51
Tang S et al (2019) Brain-targeted intranasal delivery of dopamine with borneol and lactoferrin co-modified nanoparticles for treating Parkinson’s disease. Drug Deliv 26(1):700–707
Tian Y et al (2022) Iron metabolism in aging and age-related diseases. Int J Mol Sci 23(7):3612
Tinari A et al (2005) Inhibitory activity of bovine lactoferrin against echovirus induced programmed cell death in vitro. Int J Antimicrob Agents 25(5):433–438
Tomita M et al (1991) Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J Dairy Sci 74(12):4137–4142
Trentini A et al (2020) Vaginal lactoferrin administration decreases oxidative stress in the amniotic fluid of pregnant women: an open-label randomized pilot study. Front Med (lausanne) 7:555
Tung YT et al (2013) Bovine lactoferrin inhibits lung cancer growth through suppression of both inflammation and expression of vascular endothelial growth factor. J Dairy Sci 96(4):2095–2106
Valko M et al (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40
Van Berkel PH et al (1997) N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem J 328(1):145–151
van der Strate BW et al (2001) Antiviral activities of lactoferrin. Antivir Res 52(3):225–239
van Eck NJ, Waltman L (2022). https://www.vosviewer.com . Accessed 20 June 2022
van Eck NJ, Waltman L (2010) Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84(2):523–538
Varela-Fernández R et al (2022) Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur J Pharm Biopharm 172:144–156
Vorland LH et al (1998) Lactoferricin of bovine origin is more active than lactoferricins of human, murine and caprine origin. Scand J Infect Dis 30(5):513–517
Wang B et al (2019) Lactoferrin: structure, function, denaturation and digestion. Crit Rev Food Sci Nutr 59(4):580–596
Wang Y et al (2020) Lactoferrin for the treatment of COVID-19 (Review). Exp Ther Med 20(6):272
Ward JL, Torres-Gonzalez M, Ammons MCB (2022) The influence of viral infections on iron homeostasis and the potential for lactoferrin as a therapeutic in the age of the SARS-CoV-2 pandemic. Nutrients 14(15):3090
Wolf JS et al (2007) Oral lactoferrin results in T cell-dependent tumor inhibition of head and neck squamous cell carcinoma in vivo. Clin Cancer Res 13(5):1601–1610
Wróbel M, Małaczewska J, Kaczorek-Łukowska E (2022) Antiviral effect of bovine lactoferrin against enterovirus E. Molecules 27(17):5569
Wu J-X et al (2023) Protective effect and mechanism of lactoferrin combined with hypoxia against high-fat diet induced obesity and non-alcoholic fatty liver disease in mice. Int J Biol Macromol 227:839–850
Yan R et al (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367(6485):1444–1448
Yount NY et al (2007) The gamma-core motif correlates with antimicrobial activity in cysteine-containing kaliocin-1 originating from transferrins. Biochim Biophys Acta 1768(11):2862–2872
Zapata RC et al (2017) Whey protein components - lactalbumin and lactoferrin - improve energy balance and metabolism. Sci Rep 7(1):9917
Zhang Y, Lima CF, Rodrigues LR (2014) Anticancer effects of lactoferrin: underlying mechanisms and future trends in cancer therapy. Nutr Rev 72(12):763–773
Zheng J et al (2020) Lactoferrin improves cognitive function and attenuates brain senescence in aged mice. J Funct Foods 65:103736
Zimecki M et al (2008) The concerted action of lactoferrin and bacteriophages in the clearance of bacteria in sublethally infected mice. Adv Hyg Exp Med 62:42
Zimecki M, Actor JK, Kruzel ML (2021) The potential for lactoferrin to reduce SARS-CoV-2 induced cytokine storm. Int Immunopharmacol 95:107571
Zimmermann MB (2020) Global look at nutritional and functional iron deficiency in infancy. Hematology 2020(1):471–477
Acknowledgements
EBS and KK acknowledge FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences—UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy—i4HB. FF acknowledges Laboratório Associado para a Química Verde—Tecnologias e Processos Limpos—UIDB/50006/2020 that supports her Grant REQUIMTE 2020-20.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
CC, EB, KK and EBS contributed to the conceptualization, methodology, validation, formal analysis, investigation, and writing—original draft preparation. CBL, FF, and MBPPO con-tributed to data validation, figures and tables, literature review, and software management. MBPPO, KK and EBS contributed to the writing—review and editing, project administration, resources, supervision, and funding acquisition. All authors have made a substantial contribution to the work. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Authors report no conflict of interests with respect to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coccolini, C., Berselli, E., Blanco-Llamero, C. et al. Biomedical and Nutritional Applications of Lactoferrin. Int J Pept Res Ther 29, 71 (2023). https://doi.org/10.1007/s10989-023-10541-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-023-10541-2