Abstract
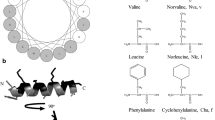
Due to increasing resistance of bacteria to traditional antibiotics, antimicrobial peptides are being investigated as a promising alternative. Tachyplesin, an antimicrobial peptide isolated from horseshoe crab, inhibits the growth of many different types of bacteria with its ability to permeabilize the cell membrane. Starting with a previously reported linear tachyplesin analog lacking cysteine (cysteine-deleted tachyplesin, CDT, KWFRVYRGIYRRR-CONH2), this study examines the systematic deletion of the C-terminal arginines and the N-terminal lysine, addition of positively charged N-and C-terminal residues, replacement of arginine with similarly-charged lysine, and replacement of hydrophobic residues with aliphatic, aromatic, fluoro-substituted aromatic, and bicyclic amino acids to examine effects on activity. The 16 modified CDT analogs were tested for their ability to disrupt model liposomes, and minimum inhibitory concentrations were determined for gram-positive and gram-negative bacterial strains. Hemolytic activity also was assessed. Overall, results indicate that elimination of two C-terminal arginine residues results in a peptide ([des-Arg12,13]CDT) with preserved antimicrobial activity but a reduction in hemolysis, a selectivity desirable for a therapeutic agent. Additional deletion was not tolerated, nor was addition of residues at the termini. Analysis of the 16 analogs also reveals the importance of hydrophobicity, not necessarily aromaticity, as an analog with hydrophobic isoleucine residues placed throughout the sequence ([Ile2,3,6,10]CDT) displayed comparable antimicrobial activity to CDT with lower hemolysis, representing a promising antimicrobial peptide with lowered toxicity.



Similar content being viewed by others
References
Alanis AJ (2005) Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res 36:697–705. doi:10.1016/j.arcmed.2005.06.009
Cho J, Lee DG (2011) The characteristic region of arenicin-1 involved with a bacterial membrane targeting mechanism. Biochem Biophys Res Commun 405:422–427. doi:10.1016/j.bbrc.2011.01.046
Dathe M, Nikolenko H, Klose J, Bienert M (2004) Cyclization increases the antimicrobial activity and selectivity of arginine- and tryptophan-containing hexapeptides. Biochemistry 43:9140–9150. doi:10.1021/bi035948v
Eckert R (2011) Road to clinical efficacy: challenges and novel strategies for antimicrobial peptide development. Future Microbiol 6:635–651. doi:10.2217/fmb.11.27
Gimenez D, Andreu C, del Olmo M et al (2006) The introduction of fluorine atoms or trifluoromethyl groups in short cationic peptides enhances their antimicrobial activity. Bioorg Med Chem 14:6971–6978. doi:10.1016/j.bmc.2006.06.027
Gottler LM, de la Salud Bea R, Shelburne CE (2008) Using fluorous amino acids to probe the effects of changing hydrophobicity on the physical and biological properties of the beta-hairpin antimicrobial peptide protegrin-1. Biochemistry (Mosc) 47:9243–9250. doi:10.1021/bi801045n
Hallock KJ, Wildman KH, Lee DK, Ramamoorthy A (2002) An innovative procedure using a sublimable solid to align lipid bilayers for solid-state NMR studies. Biophys J 82:2499–2503
Haney EF, Nguyen LT, Schibli DJ, Vogel HJ (2012) Design of a novel tryptophan-rich membrane-active antimicrobial peptide from the membrane-proximal region of the HIV glycoprotein, gp41. Beilstein J Org Chem 8:1172–1184. doi:10.3762/bjoc.8.130
Laederach A, Andreotti AH, Fulton DB (2002) Solution and micelle-bound structures of tachyplesin I and its active aromatic linear derivatives. Biochemistry (Mosc) 41:12359–12368. doi:10.1021/bi026185z
Lee K-H, Lee H-Y, Slutsky MM et al (2004) Fluorous effect in proteins: De Novo design and characterization of a four-α-helix bundle protein containing hexafluoroleucine. Biochemistry (Mosc) 43:16277–16284. doi:10.1021/bi049086p
Marsh ENG, Buer BC, Ramamoorthy A (2009) Fluorine—a new element in the design of membrane-active peptides. Mol BioSyst 5:1143–1147. doi:10.1039/b909864j
Matsuzaki K, Yoneyama S, Fujii N et al (1997) Membrane permeabilization mechanisms of a cyclic antimicrobial peptide, tachyplesin I, and its linear analog. Biochemistry (Mosc) 36:9799–9806
Merrifield RB (1963) Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J Am Chem Soc 85:2149–2154. doi:10.1021/ja00897a025
Nguyen LT, Haney EF, Vogel HJ (2011) The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol 29:464–472. doi:10.1016/j.tibtech.2011.05.001
Niemz A, Tirrell DA (2001) Self-association and membrane-binding behavior of melittins containing trifluoroleucine. J Am Chem Soc 123:7407–7413. doi:10.1021/ja004351p
Paredes-Gamero EJ, Martins MNC, Cappabianco FAM et al (2012) Characterization of dual effects induced by antimicrobial peptides: regulated cell death or membrane disruption. Biochim Biophys Acta 1820:1062–1072. doi:10.1016/j.bbagen.2012.02.015
Podorieszach AP, Huttunen-Hennelly HEK (2010) The effects of tryptophan and hydrophobicity on the structure and bioactivity of novel indolicidin derivatives with promising pharmaceutical potential. Org Biomol Chem 8:1679. doi:10.1039/b921248e
Poole K (2001) Multidrug resistance in Gram-negative bacteria. Curr Opin Microbiol 4:500–508. doi:10.1016/S1369-5274(00)00242-3
Ramamoorthy A (2009) Beyond NMR spectra of antimicrobial peptides: dynamical images at atomic resolution and functional insights. Solid State Nucl Magn Reson 35:201–207. doi:10.1016/j.ssnmr.2009.03.003
Ramamoorthy A, Thennarasu S, Tan A et al (2006a) Deletion of all cysteines in tachyplesin I abolishes hemolytic activity and retains antimicrobial activity and lipopolysaccharide selective binding. Biochemistry (Mosc) 45:6529–6540. doi:10.1021/bi052629q
Ramamoorthy A, Thennarasu S, Tan A et al (2006b) Cell selectivity correlates with membrane-specific interactions: a case study on the antimicrobial peptide G15 derived from granulysin. Biochim Biophys Acta 1758:154–163. doi:10.1016/j.bbamem.2006.02.014
Rao AG (1999) Conformation and antimicrobial activity of linear derivatives of tachyplesin lacking disulfide bonds. Arch Biochem Biophys 361:127–134. doi:10.1006/abbi.1998.0962
Rosenfeld Y, Lev N, Shai Y (2010) Effect of the hydrophobicity to net positive charge ratio on antibacterial and anti-endotoxin activities of structurally similar antimicrobial peptides. Biochemistry (Mosc) 49:853–861. doi:10.1021/bi900724x
Ryge TS, Doisy X, Ifrah D et al (2004) New indolicidin analogues with potent antibacterial activity. J Pept Res 64:171–185. doi:10.1111/j.1399-3011.2004.00177.x
Saravanan R, Mohanram H, Joshi M et al (2012) Structure, activity and interactions of the cysteine deleted analog of tachyplesin-1 with lipopolysaccharide micelle: mechanistic insights into outer-membrane permeabilization and endotoxin neutralization. Biochim Biophys Acta 1818:1613–1624. doi:10.1016/j.bbamem.2012.03.015
Saravanan R, Li X, Lim K et al (2014) Design of short membrane selective antimicrobial peptides containing tryptophan and arginine residues for improved activity, salt-resistance, and biocompatibility. Biotechnol Bioeng 111:37–49. doi:10.1002/bit.25003
Schullery SE, Mohammedshah T, Makhlouf H et al (1997) Binding to δ and μ opioid receptors by deltorphin I/II analogues modified at the phe3 and asp4/glu4 side chains: a report of 32 new analogues and a QSAR study. Bioorgan Med Chem 5:2221–2234. doi:10.1016/S0968-0896(97)00163-6
Schullery SE, Rodgers DW, Tripathy S et al (2001) The role of backbone conformation in deltorphin II binding: a QSAR study of new analogues modified in the 5-, 6-positions of the address domain. Bioorgan Med Chem 9:2633–2642. doi:10.1016/S0968-0896(01)00183-3
Staubitz P, Peschel A, Nieuwenhuizen WF et al (2001) Structure-function relationships in the tryptophan-rich, antimicrobial peptide indolicidin. J Pept Sci 7:552–564. doi:10.1002/psc.351
Tamamura H, Ikoma R, Niwa M et al (1993) Antimicrobial activity and conformation of tachyplesin-I and its analogs. Chem Pharm Bull (Tokyo) 41:978–980
Thennarasu S, Tan A, Penumatchu R et al (2010) Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL37. Biophys J 98:248–257. doi:10.1016/j.bpj.2009.09.060
Wessolowski A, Bienert M, Dathe M (2004) Antimicrobial activity of arginine- and tryptophan-rich hexapeptides: the effects of aromatic clusters, D-amino acid substitution and cyclization. J Pept Res 64:159–169. doi:10.1111/j.1399-3011.2004.00182.x
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. doi:10.1038/nprot.2007.521
Yu H-Y, Tu C-H, Yip B-S et al (2011) Easy strategy to increase salt resistance of antimicrobial peptides. Antimicrob Agents Chemother 55:4918–4921. doi:10.1128/AAC.00202-11
Acknowledgments
The authors would like to thank Dr. Ruth Ann Armitage for mass spectrometry analysis. Funding was provided by the Eastern Michigan University Undergraduate Research Stimulus Program, an Eastern Michigan University Provost’s Research Support Award and the Chemistry Department Seller’s Fund.
Conflict of interest
Stacie J. Wood, Yeji A. Park, Naga Pooja Kanneganti, Hareesh Reddy Mukkisa, Lauren L. Crisman, Sarah E. Davis, James L. Vandenbosch, Jamie B. Scaglione, and Deborah L. Heyl declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wood, S.J., Park, Y.A., Kanneganti, N.P. et al. Modified Cysteine-Deleted Tachyplesin (CDT) Analogs as Linear Antimicrobial Peptides: Influence of Chain Length, Positive Charge, and Hydrophobicity on Antimicrobial and Hemolytic Activity. Int J Pept Res Ther 20, 519–530 (2014). https://doi.org/10.1007/s10989-014-9419-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-014-9419-7