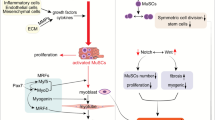
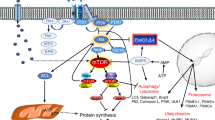
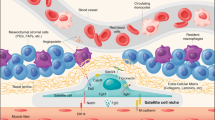
Abstract
Skeletal muscle hypertrophy is a result of increased load, such as functional and stretch-overload. Activation of satellite cells and proliferation, differentiation and fusion are required for hypertrophy of overloaded skeletal muscles. On the contrary, a dramatic loss of skeletal muscle mass determines atrophy settings. The epigenetic changes involved in gene regulation at DNA and chromatin level are critical for the opposing phenomena, muscle growth and atrophy. Physiological properties of skeletal muscle tissue play a fundamental role in health and disease since it is the most abundant tissue in mammals. In fact, protein synthesis and degradation are finely modulated to maintain an appropriate muscle mass. When the molecular signaling is altered muscle wasting and weakness occurred, and this happened in most common inherited and acquired disorders such as muscular dystrophies, cachexia, and age-related wasting. To date, there is no accepted treatment to improve muscle size and strength, and these conditions pose a considerable anxiety to patients as well as to public health. Several molecules, including Magic-F1, myostatin inhibitor, IGF, glucocorticoids and microRNAs are currently investigated to interfere positively in the blueprint of skeletal muscle growth and regeneration.


Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Acharyya S, Butchbach ME et al (2005) Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 8(5):421–432
Adams GR, McCue SA (1998) Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84(5):1716–1722
Adams GR, Caiozzo VJ et al (2002) Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 283(4):C1182–C1195
Adams GR, Caiozzo VJ et al (2003) Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol 95(6):2185–2201
Adi S, Bin-Abbas B et al (2002) Early stimulation and late inhibition of extracellular signal-regulated kinase 1/2 phosphorylation by IGF-I: a potential mechanism mediating the switch in IGF-I action on skeletal muscle cell differentiation. Endocrinology 143(2):511–516
Allen RE, Boxhorn LK (1989) Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138(2):311–315
Allen RE, Sheehan SM et al (1995) Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165(2):307–312
Amthor H, Christ B et al (2002) Follistatin regulates bone morphogenetic protein-7 (BMP-7) activity to stimulate embryonic muscle growth. Dev Biol 243(1):115–127
Amthor H, Otto A et al (2006) Myostatin imposes reversible quiescence on embryonic muscle precursors. Dev Dyn 235(3):672–680
Artaza JN, Bhasin S et al (2005) Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology 146(8):3547–3557
Aziz MH, Kumar R et al (2003) Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review). Int J Oncol 23(1):17–28
Bamman MM, Shipp JR et al (2001) Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab 280(3):E383–E390
Baracos VE (2001) Management of muscle wasting in cancer-associated cachexia: understanding gained from experimental studies. Cancer 92(6 Suppl):1669–1677
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Barton-Davis ER, Shoturma DI et al (1999) Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand 167(4):301–305
Beauchamp JR, Heslop L et al (2000) Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151(6):1221–1234
Bodine SC, Latres E et al (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294(5547):1704–1708
Bogdanovich S, Krag TO et al (2002) Functional improvement of dystrophic muscle by myostatin blockade. Nature 420(6914):418–421
Bogdanovich S, Perkins KJ et al (2005) Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. Faseb J 19(6):543–549
Brunet A, Sweeney LB et al (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303(5666):2011–2015
Buckingham M (2001) Skeletal muscle formation in vertebrates. Curr Opin Genet Dev 11(4):440–448
Cassano M, Biressi S et al (2008) Magic-factor 1, a partial agonist of Met, induces muscle hypertrophy by protecting myogenic progenitors from apoptosis. PLoS One 3(9):e3223
Chakravarthy MV, Davis BS et al (2000) IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol 89(4):1365–1379
Charlier C, Coppieters W et al (1995) The mh gene causing double-muscling in cattle maps to bovine Chromosome 2. Mamm Genome 6(11):788–792
Clarke BA, Drujan D et al (2007) The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 6(5):376–385
Clop A, Marcq F et al (2006) A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet 38(7):813–818
Coleman ME, DeMayo F et al (1995) Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270(20):12109–12116
Conboy IM, Rando TA (2002) The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell 3(3):397–409
Conboy IM, Conboy MJ et al (2003) Notch-mediated restoration of regenerative potential to aged muscle. Science 302(5650):1575–1577
Coulton GR, Curtin NA et al (1988a) The mdx mouse skeletal muscle myopathy: II. Contractile properties. Neuropathol Appl Neurobiol 14(4):299–314
Coulton GR, Morgan JE et al (1988b) The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol 14(1):53–70
Crisa A, Marchitelli C et al (2003) Sequence analysis of myostatin promoter in cattle. Cytogenet Genome Res 102(1–4):48–52
DeVol DL, Rotwein P (1990) Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol 259(1 Pt 1):E89–E95
Dhawan J, Rando TA (2005) Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15(12):666–673
Di Giovanni S, Molon A et al (2004) Constitutive activation of MAPK cascade in acute quadriplegic myopathy. Ann Neurol 55(2):195–206
Doumit ME, Cook DR et al (1996) Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology 137(4):1385–1394
Ebisawa T, Fukuchi M et al (2001) Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem 276(16):12477–12480
Fiaccavento R, Carotenuto F et al (2005) Stem cell activation sustains hereditary hypertrophy in hamster cardiomyopathy. J Pathol 205(3):397–407
Fiorotto ML, Schwartz RJ et al (2003) Persistent IGF-I overexpression in skeletal muscle transiently enhances DNA accretion and growth. Faseb J 17(1):59–60
Forte G, Minieri M et al (2006) Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells 24(1):23–33
Galvez BG, Sampaolesi M et al (2006) Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol 174(2):231–243
Gargioli C, Coletta M et al (2008) PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med 14(9):973–978
Gomes MD, Lecker SH et al (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98(25):14440–14445
Greer EL, Brunet A (2005) FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24(50):7410–7425
Guttridge DC, Mayo MW et al (2000) NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289(5488):2363–2366
Haddad F, Adams GR (2002) Selected contribution: acute cellular and molecular responses to resistance exercise. J Appl Physiol 93(1):394–403
Haddad F, Adams GR (2004) Inhibition of MAP/ERK kinase prevents IGF-I-induced hypertrophy in rat muscles. J Appl Physiol 96(1):203–210
Hameed M, Lange KH et al (2004) The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol 555(Pt 1):231–240
Hannan KM, Thomas G et al (2003) Activation of S6K1 (p70 ribosomal protein S6 kinase 1) requires an initial calcium-dependent priming event involving formation of a high-molecular-mass signalling complex. Biochem J 370(Pt 2):469–477
Hardt SE, Sadoshima J (2002) Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res 90(10):1055–1063
Heslop L, Morgan JE et al (2000) Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci 113(Pt 12):2299–2308
Hill M, Goldspink G (2003) Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549(Pt 2):409–418
Hill JJ, Qiu Y et al (2003) Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol 17(6):1144–1154
Horsley V, Jansen KM et al (2003) IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113(4):483–494
Hribal ML, Nakae J et al (2003) Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J Cell Biol 162(4):535–541
Huang J, Forsberg NE (1998) Role of calpain in skeletal-muscle protein degradation. Proc Natl Acad Sci USA 95(21):12100–12105
Husmann I, Soulet L et al (1996) Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev 7(3):249–258
Iemura S, Yamamoto TS et al (1998) Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci USA 95(16):9337–9342
Joulia D, Bernardi H et al (2003) Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res 286(2):263–275
Kamei Y, Miura S et al (2004) Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279(39):41114–41123
Langenbach KJ, Rando TA (2002) Inhibition of dystroglycan binding to laminin disrupts the PI3 K/AKT pathway and survival signaling in muscle cells. Muscle Nerve 26(5):644–653
Langley B, Thomas M et al (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277(51):49831–49840
Lapidos KA, Kakkar R et al (2004) The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res 94(8):1023–1031
Latres E, Amini AR et al (2005) Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3 K/Akt/mTOR) pathway. J Biol Chem 280(4):2737–2744
Lecker SH, Solomon V et al (1999) Ubiquitin conjugation by the N-end rule pathway and mRNAs for its components increase in muscles of diabetic rats. J Clin Invest 104(10):1411–1420
Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98(16):9306–9311
Lee CT, Risom T et al (2007) Evolutionary conservation of microRNA regulatory circuits: an examination of microRNA gene complexity and conserved microRNA-target interactions through metazoan phylogeny. DNA Cell Biol 26(4):209–218
Li YP, Reid MB (2001) Effect of tumor necrosis factor-alpha on skeletal muscle metabolism. Curr Opin Rheumatol 13(6):483–487
Li YP, Lecker SH et al (2003) TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J 17(9):1048–1057
Li Y, Takemura G et al (2003) Postinfarction treatment with an adenoviral vector expressing hepatocyte growth factor relieves chronic left ventricular remodeling and dysfunction in mice. Circulation 107(19):2499–2506
Li ZF, Shelton GD et al (2005) Elimination of myostatin does not combat muscular dystrophy in dy mice but increases postnatal lethality. Am J Pathol 166(2):491–497
Li F, Zhang C et al (2005) ANG II-induced neointimal growth is mediated via cPLA2- and PLD2-activated Akt in balloon-injured rat carotid artery. Am J Physiol Heart Circ Physiol 289(6):H2592–H2601
Ma K, Mallidis C et al (2001) Characterization of 5′-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab 281(6):E1128–E1136
Machida S, Spangenburg EE et al (2003) Forkhead transcription factor FoxO1 transduces insulin-like growth factor’s signal to p27Kip1 in primary skeletal muscle satellite cells. J Cell Physiol 196(3):523–531
Massague J, Chen YG (2000) Controlling TGF-beta signaling. Genes Dev 14(6):627–644
Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–495
McCarthy JJ, Esser KA et al (2007) MicroRNA-206 is overexpressed in the diaphragm but not the hindlimb muscle of mdx mouse. Am J Physiol Cell Physiol 293(1):C451–C457
McCroskery S, Thomas M et al (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162(6):1135–1147
McCroskery S, Thomas M et al (2005) Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci 118(Pt 15):3531–3541
McElhinny AS, Kakinuma K et al (2002) Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol 157(1):125–136
McGeachie JK, Grounds MD (1987) Initiation and duration of muscle precursor replication after mild and severe injury to skeletal muscle of mice. An autoradiographic study. Cell Tissue Res 248(1):125–130
McPherron AC, Lawler AM et al (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387(6628):83–90
McPherron AC, Lee SJ (2002) Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109(5):595–601
Minetti GC, Colussi C et al (2006) Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med 12(10):1147–1150
Miro O, Pedrol E et al (1997) Skeletal muscle studies in patients with HIV-related wasting syndrome. J Neurol Sci 150(2):153–159
Mitch WE, Price SR (2001) Transcription factors and muscle cachexia: is there a therapeutic target? Lancet 357(9258):734–735
Motta MC, Divecha N et al (2004) Mammalian SIRT1 represses forkhead transcription factors. Cell 116(4):551–563
Musaro A, McCullagh K et al (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27(2):195–200
Nakamura T, Takio K et al (1990) Activin-binding protein from rat ovary is follistatin. Science 247(4944):836–838
Nowak KJ, Davies KE (2004) Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment. EMBO Rep 5(9):872–876
O’Rourke JR, Georges SA et al (2007) Essential role for Dicer during skeletal muscle development. Dev Biol 311(2):359–368
Phelan JN, Gonyea WJ (1997) Effect of radiation on satellite cell activity and protein expression in overloaded mammalian skeletal muscle. Anat Rec 247(2):179–188
Price DA, Bassendine MF et al (2006) Apolipoprotein epsilon3 allele is associated with persistent hepatitis C virus infection. Gut 55(5):715–718
Putman CT, Dusterhoft S et al (1999) Changes in satellite cell content and myosin isoforms in low-frequency-stimulated fast muscle of hypothyroid rat. J Appl Physiol 86(1):40–51
Reimann J, Brimah K et al (2004) Pax7 distribution in human skeletal muscle biopsies and myogenic tissue cultures. Cell Tissue Res 315(2):233–242
Reisz-Porszasz S, Bhasin S et al (2003) Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab 285(4):E876–E888
Rios R, Carneiro I et al (2002) Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol 282(5):C993–C999
Roberts SB, Goetz FW (2001) Differential skeletal muscle expression of myostatin across teleost species, and the isolation of multiple myostatin isoforms. FEBS Lett 491(3):212–216
Rosenblatt JD, Yong D et al (1994) Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve 17(6):608–613
Rudnicki MA, Le Grand F et al (2008) The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73:323–331
Sacheck JM, Ohtsuka A et al (2004) IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287(4):E591–E601
Saitoh M, Pullen N et al (2002) Regulation of an activated S6 kinase 1 variant reveals a novel mammalian target of rapamycin phosphorylation site. J Biol Chem 277(22):20104–20112
Salerno MS, Thomas M et al (2004) Molecular analysis of fiber type-specific expression of murine myostatin promoter. Am J Physiol Cell Physiol 287(4):C1031–C1040
Sampaolesi M, Torrente Y et al (2003) Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science 301(5632):487–492
Sampaolesi M, Biressi S et al (2005) Cell therapy of primary myopathies. Arch Ital Biol 143(3–4):235–242
Sampaolesi M, Blot S et al (2006) Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444(7119):574–579
Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23:160–170
Sandri M, Sandri C et al (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117(3):399–412
Sartorelli V, Fulco M (2004) Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci STKE 2004(244):re11
Schakman O, Gilson H et al (2008) Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 197(1):1–10
Schiaffino S, Sandri M et al (2007) Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 22:269–278
Schuelke M, Wagner KR et al (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350(26):2682–2688
Secrist JP, Zhou X et al (2003) HDAC inhibitors for the treatment of cancer. Curr Opin Investig Drugs 4(12):1422–1427
Seuntjens E, Umans L et al (2009) Transforming Growth Factor type beta and Smad family signaling in stem cell function. Cytokine Growth Factor Rev
Sever JL, Rakusan TA et al (1996) HIV antibody responses in children of HIV-infected mothers. Pediatr AIDS HIV Infect 7(4):246–253
Sherwood RI, Christensen JL et al (2004) Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell 119(4):543–554
Singh JP, Larson MG et al (2001) Genetic factors contribute to the variance in frequency domain measures of heart rate variability. Auton Neurosci 90(1–2):122–126
Southgate RJ, Bruce CR et al (2005) PGC-1alpha gene expression is down-regulated by Akt- mediated phosphorylation and nuclear exclusion of FoxO1 in insulin-stimulated skeletal muscle. Faseb J 19(14):2072–2074
Southgate RJ, Neill B et al (2007) FOXO1 regulates the expression of 4E-BP1 and inhibits mTOR signaling in mammalian skeletal muscle. J Biol Chem 282(29):21176–21186
Spiller MP, Kambadur R et al (2002) The myostatin gene is a downstream target gene of basic helix-loop-helix transcription factor MyoD. Mol Cell Biol 22(20):7066–7082
Stark A, Brennecke J et al (2005) Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3′UTR evolution. Cell 123(6):1133–1146
Stitt TN, Drujan D et al (2004) The IGF-1/PI3 K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14(3):395–403
Suzuki N, Motohashi N et al (2007) NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest 117(9):2468–2476
Tran H, Brunet A et al (2003) The many forks in FOXO’s road. Sci STKE 2003(172):RE5
Voisin L, Gray K et al (1996) Altered expression of eukaryotic initiation factor 2B in skeletal muscle during sepsis. Am J Physiol 270(1 Pt 1):E43–E50
Wagner KR, McPherron AC et al (2002) Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 52(6):832–836
Wagner KR, Liu X et al (2005) Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA 102(7):2519–2524
Wehling M, Cai B et al (2000) Modulation of myostatin expression during modified muscle use. Faseb J 14(1):103–110
Whalen RG, Harris JB et al (1990) Expression of myosin isoforms during notexin-induced regeneration of rat soleus muscles. Dev Biol 141(1):24–40
Yang ZZ, Tschopp O et al (2004) Physiological functions of protein kinase B/Akt. Biochem Soc Trans 32(Pt 2):350–354
Zammit P, Beauchamp J (2001) The skeletal muscle satellite cell: stem cell or son of stem cell? Differentiation 68(4-5):193–204
Zammit PS, Relaix F et al (2006) Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci 119(Pt 9):1824–1832
Zhu X, Topouzis S et al (2004) Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine 26(6):262–272
Acknowledgment
Our work is supported by grants from FWO Odysseus Program n. G.0907.08; Wicka Funds n. zkb8720; the Italian Ministry of University and Scientific Research (grant n. 2005067555_003, PRIN 2006–08), Association Francoise contre les Myopathies, FP7 CARE-MI n.242038 and CARIPLO Foundation (grants n. 2007.5639 2008.2005). We are grateful to Catherine Verfaillie, Giulio Cossu and Danny Huleybrook for continuous support and Gianpaolo Papaccio for helpful discussion. We thank, Christina Vochten and Luigi Vercesi for the professional secretarial service, and Paolo Luban for a kind donation. We apologize to colleagues whose work could not be cited due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cassano, M., Quattrocelli, M., Crippa, S. et al. Cellular mechanisms and local progenitor activation to regulate skeletal muscle mass. J Muscle Res Cell Motil 30, 243–253 (2009). https://doi.org/10.1007/s10974-010-9204-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-010-9204-y
Keywords
Profiles
- Marco Cassano View author profile