Abstract
The calorimetric and DTA/TG measurements were applied in testing the effect of granulated blast furnace slag originated from the storage yards of different age, added as a supplementary cementing material to the Portland cement clinker. The studies were performed with aim to evaluate the kinetics of cement hydration and the modification of hydrated paste composition in the presence of additive. The material after 20-year storage, the crushed slag after approximately 2-years storage and the new slag from the current production in the metallurgical plant were used. The slag percentage was 5 ÷ 50%. The addition of granulated blast furnace slag stored for a long time affects the standard properties of cement reducing the compressive strength at longer maturing and with the percentage of additive. This is related mainly to the reduction in the vitreous component. However, at the additive content up to 50% the binder complying with the requirements of the relevant European standards for common cements could be produced. Basing on the results of TG measurements the role of calcium carbonate, being the product resulting from the slag weathering process, acting as a grindability and setting/hardening modifying agent, was highlighted.
Similar content being viewed by others
Introduction
As it is commonly known in cement chemistry, the calorimetric measurements give the possibility to follow the changes occurring in the hydrating mixture produced at standard water to cement ratio in a continuous way from the beginning of the process, without removal of water (e.g., for the XRD or DTA studies the so-called chemical drying should be done and the data obtained versus time are of discrete character). The heat evolved versus time curves reflect very precisely the progress of complex phenomena and reactions with water, accompanying the increasing content of hydration product. After the initial intensive heat evolution during the first minutes (water adsorption and surface dissolution) the heat evolution decreases in so-called dormant period and subsequently the heat evolution (corresponding to the growth of hydration products) increases steadily and a maximum is attained. The increase in total heat evolved values becomes very slow after 2–3 days when the layer of products is formed on cement grains and the process is diffusion controlled [e.g.,1]. According to the standards, the values after 41 h from the processing with water are measured and evaluated. The calorimetric measurements and generally the thermoanalytical techniques were always a significant base in the discussion of the impact of several factors modifying the hydration process. It relates particularly to the use of supplementary materials. Some contributions have been published in the Journal of Thermal Analysis quite recently [2,3,4,5,6,7,8,9].
The production of Portland cement clinker accounts for up to 6–7% of global CO2 anthropogenic emission [10,11,12,13,14], contributing to the global warming, natural resource depletion and an intensive energy demand. The problems of CO2 reduction in cement industry “on the way to low carbon future” have been discussed in many works, for example in the general report presented by Schneider during the International Conference on Cement Chemistry in Prague [15]. In the light of new EU regulations the carbon dioxide emissions in cement plants must be reduced by 30 percentage points by 2030, as compared to the level in 2005. Therefore, the cement manufacturers should look for cements with a low so-called clinker factor [16,17,18].
The European average clinker factor indicating the Portland cement clinker replacement by the so-called supplementary cementing materials (SCM) was 0.74 in 2016 [19], similarly as in in Poland (0.75) [20,21,22].
Cement industry uses waste from other industries as SCMs as well as the alternative fuels in the production process [15, 23]. The replacement of Portland cement clinker by SCMs has been an important research area for many decades, subsequently adopted in the industrial practice [24,25,26,27].
Ground granulated blast furnace slag (GGBFS) from pig iron production is among the most commonly used SCMs [15, 24,25,26,27,28], substituting even up to 95% Portland cement clinker in case of CEM III C according to the European 197–1 standard [28, 29]. The use of granulated blast furnace slag as cement constituent is of great importance not only due to its high performance as compared to the other supplementary cementing materials but also as a durability enhancing component [30,31,32,33,34,35].
Ground granulated blast furnace slag from the pig iron production is a vitreous by-product derived from the smelting of metallic ores. The granulation of molten slag is obtained by rapid cooling preventing the crystallization and producing a granular glassy material that after grinding reveals good hydraulic properties making it a Portland clinker substitute. Many research projects have been devoted to the role of GGBFS for over 50 years [32,33,34,35,36].
The amount of blast furnace slag available globally is only around 330 Mt/year, and despite the growth in steel production, the production of iron and slag is expected to diminish. More than 90% of blast furnace slag is already used as either as cement component added in cement plants or as a component of concrete [11]. Moreover, the proportion of SCMs can be increased significantly as it results from the revised European cement standard EN 197–1 (so-called CEM II/C and CEM VI common cements) [28, 37].
However, though the lower clinker factors are recommended and possible, the regional availability of basic supplementary cement materials has become the limiting factor. This relates particularly to the availability of slag type material. The limited availability can make difficult to get sufficient amount of GGBFS for cement production [38, 39]. In Poland the supply of slag is significantly smaller than the demand, hence there are some problems related to the shortage of this material [20, 21].
While many publications have dealt with the use of fresh-made granulated blast furnace slag as a cementitious material, there are only a few published reports about the use of weathered slag [40, 41]. The authors found generally a loss of 28-day compressive strength and an increase in setting times of cements containing “old” slag. Simultaneously, an improvement of weathered slag grindability was reported. It rather seems that the results of detailed studies dealing with the implementation of the old, recovered materials are not commonly available.
The reactivity of slag is due to both its characteristics and to some external factors. For practical purposes and quality control the chemical composition and glass content are taken into account; the glass content decreases with time of storage due to the partial devitrification of slag but this is not the only detrimental phenomenon. The characteristics of slag are related to the operational conditions of blast furnace, temperature and viscosity of molten slag, granulation process and installation, etc. [42, 43]. External factors are imposed to slag by means of handling, storage and grinding.
Slag weathering results in the surface hydration, further carbonation and oxidation process that bring about the loss of slag reactivity. By comparing the cements with weathered and new slags originating from one source, a decrease in heat of hydration as well as a decrease in strength development for the materials with weathered slag are observed [41]. In order to valorize the slag deposits and the abandoned landfills, the special dryers have been installed in cement plants.
The present study relates to the properties of cements with slag originated from the storage yards of different age, added as a supplementary cementing material. These slags were examined in order to apply them on a large-scale production. In the earlier report, unpublished yet [44] it has been found that the blast furnace slags from one source, regardless of the age of storage, can be the active supplementary cementing materials, complying with the standard requirements relating to the compressive strength, initial setting time and soundness. The statistical analysis was carried out to find a relationship between the content of slag and the strength development of cements. At the significance level 0.05 at which the analysis was carried out, a close convergence of results for the 2- and 7-day strength was found. The statistical analysis of variance of cement strength shows that there are no differences, as the early strength is concerned (2-day and 7-day curing), between the series of cement containing the same quantity of slag but of different origin. However, the samples after standard 28-day maturing show well documented compressive strength decrease, regarding their composition and slag “age.”
The standard measurements were accompanied by the experiments relating to the kinetics of hydration and characterization of hydration products with aim to explain the difference between the “new” and “old” slag additive differing with structure and phase assemblage. The effect of lowered vitreous phase content and the presence of some weathered substance was investigated. The role of calcium carbonate, being the “late” product, resulting from the slag weathering process, acting as a grindability and setting/hardening modifying agent was discussed.
Experimental
Methods
The chemical composition of slag, clinker and gypsum was determined with the help of XRF spectrometer equipped with a goniometer Thermo model ARL9800-043. In the measurements of chemical composition, a pearl test on the Vulcano VAAMM fusion machine was applied. The loss of ignition, the content of chlorides, free lime, sulfates, calcium oxide and other oxides, and also the insoluble residue were analyzed in accordance with the EN 196–2:2005 standard. The vitreous phase content in slags was examined with help of microscopic method, on the basis of Polish PN-B-19707:2013–10 standard. The FTIR data were collected with help of MIR and FIR Brucker Vertex 70 V spectrometer in the range 8000–370 and 700–30 cm−1.
Specific surface area of cement was determined according to the European Standard EN 196–6:2010 “Methods of testing cement—Part 6: Determination of fineness.” The compressive strength tests were carried out in accordance with the procedure described in EN 196–1: 2006.
The investigation of hydration kinetics was done with the non-diabetic—non-isothermal differential calorimeter BMR constructed by the Institute of Physical Chemistry, Polish Academy of Sciences and further modified in the Department of Building Materials, Faculty of Material Science and Ceramics AGH. This method is very sensitive and the small samples (binder—5 g; mixed with 2.5 g distilled water; w/c = 0.5 g respectively) can be subjected to the measurements. The samples were placed in the polyethylene string bags, subsequently an appropriate amount of water was added and the pastes were homogenized (about 10 s), and then immediately placed in the measuring chamber of the apparatus. The rate of heat evolution was measured versus time, and the data were calculated. Water in these experiments was distilled and cooled in a closed container just before the start of the test to avoid carbonation.
Due to the lack of European standards specifying the method of testing chemical shrinkage, the measurements were made on the basis of ASTM C 1608–07 standard. This method consists in monitoring the change in the height of the water column in the capillaries with water placed above the container with cement paste, due to the absorption of water through the hydrating cement paste. The adsorbed water content is then converted according to the formulas given in the standard. The amount of absorbed water versus time reflects approximately the rate of hydration.
The hydrated samples were crushed, ground with acetone and dried to produce the specimens for differential thermal analysis, thermogravimetry and evolved gas analysis. The data were obtained as a function of temperature by a simultaneous Thermal Analyser STA 449F3 Jupiter (Netzsch). Around 40 mg of sample powder was placed into the corundum crucible. Samples were heated in the synthetic air atmosphere, at the flow rate 40 mL min−1, at the heating rate 15 K min−1 in the range from 40 to 1000 °C.
Materials
Clinker—the material was collected directly from the current production in cement plant in Poland. The samples were collected every 2 h for a period of 4 days. Then the separated sample of material was homogenized and reduced by coning and quartering method. The total amount of material for testing was 100 kg.
Three types of granulated blast-furnace slag were prepared for testing. Firstly, granulated slag from current production was used from the one pig iron and steel producing plant in Poland—denoted as “new” (N). Then, the slag stored on the company's waste heap, collected over a period of about two years—denoted as “crushed” (K). The last sample was prepared of blast furnace slag stored in the heap for about 20 years—the “old” slag (S). Second and third slags were pre-crushed in a jaw crusher. The slag materials were charged randomly from deliveries. Three samples from a cone of material were taken from each selected car. Then all slag samples of given type were homogenized and reduced using the quartering method. Each sample was then dried in a laboratory dryer for 12 h at 80 °C.
Natural gypsum was collected from deliveries to the cement plant. The material was then crushed in a laboratory mortar, dried in a laboratory drier at 45 °C for a period of 12 h, homogenized and a representative part was separated by quartering. The chemical composition of slags and clinker is shown in Table 1 (Fig. 1).
Physical characteristics of slags
The vitreous component content in slags was determined by microscopic method; the results are presented in Table 2. The nature of slags was also studied with help of XRD and FTIR methods; the results are illustrated as Figs. 2 And 3.
Blast furnace slags, regardless of the age of storage, are the materials with high vitreous phase content (Table 2). Correspondingly: for new slag (N)—95.7%, crushed (K) – 80.4%, and old (S)—69.9%. Concentrations of silica and alumina oxides (SiO2 + Al2O3) are: 48.2%, 48.0% and 45.8%, respectively.
XRD patterns of slags show a very high, broadened “shoulder” attributed to the amorphous nature of dominating component—the vitreous phase. However, the residue of crystalline phases: quartz and calcite are also detected in the “elder” slags. The XRD pattern for old slag (S) represents more intensive peaks at 27.5; 29.5°2θ for quartz and 33.5; 39.6°2θ for calcite. Also some low peaks can be noticed in the XRD pattern of crushed slag (K). The processes occurring during the time of storage are thus proved. The products of hydration and subsequent carbonation in these slags are the result of the presence of water (residual water from the granulation of slag and also from the rain and ground) which allows to leach out Ca(OH)2 and then react with carbon dioxide. The calcium carbonate contents in slag samples, as determined by thermogravimetric analysis, were as follows: 1.8% for the new slag (N), 4.4% for the old slag (S) and 2.5% for the crushed material (K), respectively. The amount of water was between 0.89% (N), through 1.03% (K) to 2.14% (S). The set of EGA curves (Fig. 2), related to the thermoanalytical measurements, illustrating the evolution of water vapor and carbon dioxide, shows the differences between the slags.
Slags have a diversified glassy phase structure. An increased degree of ordering in the sample of the "old" slag (S), which can be caused not only by the devitrification over longer period of time but presumably by a slightly different process of production (Fig. 3) can be observed. This is evidenced particularly by the emerging new bands in the IR spectrum attributed to the vibrations of silicon groups (at 1200 cm−1), as well as by the presence of crystalline silica found by XRD (Fig. 1). The most important bands are those attributed to the Si–O–Si and Si–O–Al bonds. All the absorption bands at 1300–850 cm−1 present in the spectra which are typical for aluminosilicates structures and assigned to the asymmetric stretching vibration of Si–O–Si and Si–O–Al bond in [SiO4]4− and [AlO4]5−. The study of the IR spectra reveals the growth of adsorbed water content, proved by the presence of bands the area of wavenumber 1600 = 1650 cm−1 and for regions 3300–3450 cm−1 (hydrogen bonded O–H).
The composition of blended cements produced using Portland cement clinker, slags and gypsum is given in Table 3.
The mixtures were ground 30 min in the laboratory mill to attain the specific surface area around 3800 cm2 g−1 (Blaine). Standard deviation of surface measurements was 245 cm2 g−1. The slag cements produced with “old” slag exhibited slightly higher specific surface; a relatively better grindability was thus proved. This is in accordance with the data reported previously [40, 41].
In order to explain some differences between the hydrated cement—slag samples the series of synthetic alite—slag mixtures was prepared. Alite is the main component of cement, being the solid solution of calcium silicate phase (Ca3[SiO4]O) [1]. Synthetic alite—tricalcium silicate doped with Al2O3 and MgO, was produced from calcium carbonate and silica-gel by calcination at 1000 ºC and subsequent repeated heating at 1500ºC, followed by grinding to the appropriate fineness.
Results and discussion
Calorimetric studies
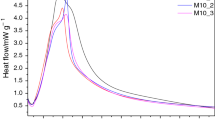
The results of calorimetric studies are presented in Figs. 4, 5, 6 as the rate of heat evolution versus time plots and total heat evolved versus time plots.
As it results from the measurements (Figs. 4–6), the addition of granulated blast furnace slag has a significant effect on the course of heat release rate curves—Q/dt = f(t). Comparing the microcalorimetric curves for cement pastes containing slag, it can be concluded that the hydration kinetics slows down generally when the percentage of granulated blast-furnace slag increases over 10% substitution. However, it can be also observed that the addition of 5% or even 10% of slag does not bring about significant change in the calorimetric curve in case of “newer” slag additive—curves are close to the curves for the CEM I reference sample. An accelerating effect of old slag additive at 5% substitution, perhaps due to the presence of fine fraction of slag, is also visible (Fig. 6). This observation may suggest that the slag grains act as nuclei on the surface of which the hydration products (C–S–H, calcium hydroxide, ettringite and other phases) are precipitated. The addition of higher amounts of slag (> 10%) causes a slight shift of the maximum heat release. It can be observed that the maximum for the sample containing 50% of the additive occurs earlier.
The total heat released decreases with increasing amount of slag both from current production (N) and slags from older heaps (see the “b” figures of calorimetric plots). For the reference sample, the heat evolved after 24 h and 40 h is 257 kJ kg−1 and 315 kJ kg−1, respectively. However, the heat calculated per 1 g of “neat” cement (clinker + gypsum) is higher for each slag containing sample, reaching 452 kJ kg−1 (72% growth) for the cement with substitution of 50% with new slag versus the value for reference sample, or 398 kJ kg−1 (64%) in case of the old slag. This proves that the slag additive is not an inert material, but it takes part in exothermic chemical reactions.
Calorimetric data (Figs. 4–6) indicate generally the active participation of slag in the hydration process. Slag grains act as the nuclei of hydration products and because of the presence of lime and glassy silica—alumina component they participate both in the hydration and pozzolanic reactions. 5% of the additive acts as a cement substitute; with increasing content in the binder, the effect of "diluting" the active material with the less active one is clearly visible. The effects seem to be a little weaker with the age of additive.
The impact of slag additive: “neat” cement substitution at low percentage and the rate of heat evolution lowering at growing dosage was proved in the measurements of heat evolved in the alite—slag mixtures with up to 50% slag (Fig. 7). As a reference sample the alite paste with no additive was taken. One can see that the slag additive plays a role of alite replacement at the dosage 5% and even at 20%.The heat evolution is weaker in the samples with 50% slag, however the hampering effect of the old slag seems to be less pronounced. The impact of slag additive on cement and alite hydration heat evolution is visualized on the common graph (Fig. 8). One can find that the retarding effect of the “old” slag is much less visible in the case of alite than in cement—the total heat evolved is higher in the presence of the old slags. Perhaps some components of weathered slag play an activating role particularly in the hydration of silicate phase at early age. One cannot exclude that the “nucleation” effect (calcium carbonate crystals, precursors of calcium silicate hydrate C–S–H) is more significant in the formation of alite hydration products.
Generally, one can conclude that the slag additive from one source, regardless of its age of storage, is not an inert material but takes part in exothermic chemical reactions in the basic environment of silicate or cement paste. Slag particles are acting as the nuclei for precipitation/crystallizations of C–S–H and calcium hydroxide, apart from the pozzolanic effect, it means the reaction of slag vitreous phase with calcium hydroxide leached from clinker components at later age.
Chemical shrinkage
The volume of cement paste is changing continuously due to the reactions which occur in cement paste. Changes at early age can have reversible character, but some are irreversible. There are several elements contributing to the phenomenon of hydration shrinkage of cement. In the initial stage of hydration, the so-called contraction takes place due to the consumption of water by hydration products in which a reduction in the volume of products compared to the volume of substrates is observed. Then, the so-called plastic shrinkage occurs, which is associated with drying and formation of a rigid structure [1]. In spite of the complex character of shrinkage phenomena, the contraction has been used as a simple method in the evaluation of water consumption progress during the first hours of the process.
The data related to the 5% and 50% slag addition are presented in Fig. 9. The shrinkage values stabilized after ca. 40 h are presented both for the slag—cement pastes (Fig. 9 top) and calculated to 1 g of cement clinker (lower illustration). The samples with 5% slag show a little higher shrinkage values at the presence of the new and crushed slag but lower water consumption for the sample with the old slag. At 50% slag dosage the shrinkage data are a little lower. However, the shrinkage values calculated for 1 g of neat cement clinker content (Fig. 9 bottom illustration) are the lowest for the reference cement paste and significantly grow at 50% slag addition. On the basis of the data presented in Fig. 9, one can conclude that the slag addition behaves as a water consumption stimulating agent and speeds up the hydration process at early age. This can indicate not only for the active participation of slag in the hydration process but also presumably for the higher water content in the product formed at higher slag addition. This is seen particularly in case of the old slag. The lowest shrinkage observed in case of cements with 50% slag additive is the consequence of the shortage of cement clinker and particularly the calcium aluminate component substantial decrease (the reaction of aluminate hydration is responsible for strong water binding in crystal structure of ettringite).
Furthermore, the analysis of chemical shrinkage results shows that the binders containing slag additive are characterized by lower change in volume. The comparison of the chemical shrinkage values (Fig. 9) shows that 50% of slag addition reduces shrinkage from 0.025 to 0.019 mL g−1 for new slag 0.021 for crushed and 0.023 for old slag.
Summary of DTA/TG/DTG/EGA results
The results of DTA measurements were very typical for the hydrated cement pastes. The peaks corresponding to the dehydration of C–S–H, ettringite and gypsum residue were found on the DTA curves of all samples in the range up to 200 °C. They become more sharp in the case of samples hydrated for at least 7 days and after 28-day hydration. Subsequently, the peak attributed to the calcium hydroxide dehydration appears in the range up to 500 °C and the peak attributed to the decarbonation—above 800 °C. The results of thermogravimetric measurements, different for particular series of cement—slag pastes are presented in Figs. 10, 11, 12.
In the reference sample with no additive the content of Ca(OH)2 and CaCO3 is 18.3%/2.5%; 24.6%/3.2% and 29.5/3.4% after 2, 7 and 28 days, respectively. A similar growth is found in case of 5% new slag additive (Figs. 10, 11, 12); however, in the samples with 5% of old slag the calcium hydroxide content and particularly the calcium carbonate are generally significantly higher. A similar trend is observed in the case of the samples including more slag. The (de)carbonation is clearly visible particularly in the case of old slag (S) higher percentage; one should remember that the sample of old slag contains initially 4.4% CaCO3. Calcium carbonate content is rising only slightly with time of hydration—and is around two times higher than in reference paste or even in the sample with the new, poorly carbonated slag.
The lowered content of Ca(OH)2 in the mixtures with growing slag content, due to the pozzolanic reaction of alumino-silicate glass is observed. The differences are not significant as in the case of typical pozzolana since these slags contain CaO. Ca(OH)2 in the pastes is rising with time of hydration; it means that the pozzolanic reaction up to 28- day maturing is not so advanced.
Replacing the new slag of better activity by materials stored for a long time does not affect significantly the calcium hydroxide content in the pastes and does not indicate for advanced pozzolanic reaction. A little lower calcium hydroxide and calcium carbonate percentage with the “age” of slag could be attributed rather to the lower vitreous phase content in these slags.
It seems that the strength decrease [44] and the heat evolved reduction which is not proportional to the lowered clinker ratio but much less pronounced, can be related to the increasing carbonate content too. The role of calcium carbonate, as a strength modifying agent in the older slag containing materials could be thus proved. The effect of calcium carbonate grains acting as hydration accelerating nuclei or improving the structure was reported in many reports [45,46,47].
Conclusions
From the results presented above the following practical conclusions can be drawn:
-
1.
Slags originating from the heaps of different age have a diversified alumino-silicate vitreous structure, with an increased degree of ordering in the samples stored for a long time. The IR spectra are typical for aluminosilicates structures. The crystalline phases: quartz and calcite are detected by XRD in the old slag.
-
2.
The water content in slags results from the surface hydration (weathering); the maximum 2.14% is found in the old slag. The release of carbon dioxide, growing with the age of slag is the consequence of hydration products carbonation; the carbon dioxide content in the old slag is 4.4,%. These values are ca. 2.5 times higher than for the material from current production.
-
3.
The hydration products in the hydrated cement—slag pastes, deduced from DTA/TG/EGA data are as follows: calcium silicate hydrate (C-S–H) and calcium sulfoaluminate—ettringite; calcium hydroxide—portlandite; calcium carbonate “initial” and detected as a product of carbonation.
-
4.
Analysis of the heat evolution during hydration of slag cements shows that the 5% substitution of clinker by slag does not affect the amount of heat released. The introduction of slag in the amount of up to 50% allows to decrease the total heat evolved by the slag cement pastes. However the total heat reduction is much less that the cement clinker substitution: by 27% for the new slag, 35% for the crushed slag and 36% for the old slag, comparing to the reference cement with no additive. An active participation of slag components in the formation of hydration products is thus proved.
-
5.
The amount of chemically bound water per 1 g of clinker is generally higher for the paste with slag, as comparing to the reference paste (chemical shrinkage tests).
-
6.
The slag particles play a role of nucleating agents; the pozzolanic effect of slag glassy phase and carbon dioxide as hydration modifying admixture cannot be excluded in mitigating the “dissolution” effect of slag component in cements.
References
Kurdowski W. Cement and concrete chemistry. Berlin:Springer 2014. ISBN-13: 9789402405941.
Palou M, Boháč M, Kuzielová E, et al. Use of calorimetry and thermal analysis to assess the heat of supplementary cementitious materials during the hydration of composite cementitious binders. J Therm Anal Calorim. 2020;142:97–117. https://doi.org/10.1007/s10973-020-09341-3.
Kuzielová E, Žemlička M, Novotný R, Palou MT. Simultaneous effect of silica fume, metakaolin and ground granulated blast-furnace slag on the hydration of multicomponent cementitious binders. J Therm Anal Calorim. 2019;136(4):1527–37. https://doi.org/10.1007/s10973-018-7813-7.
Antonovič V, Sikarskas D, Malaiškienė J, et al. Effect of pozzolanic waste materials on hydration peculiarities of Portland cement and granulated expanded glass-based plaster. J Therm Anal Calorim. 2019;138:4127–37. https://doi.org/10.1007/s10973-019-08464-6.
Malaiškienė J, Banevičienė V, Boris R, et al. The effect of dried paper-mill sludge on cement hydration. J Therm Anal Calorim. 2019;138:4107–18. https://doi.org/10.1007/s10973-019-08587-w.
Trusilewicz L, Nocuń-Wczelik W, Górak P, Woyciechowski P. Early hydration calorimetric study of the sewage sludge incinerated waste streams Portland cement-based binders: technological implications. J Therm Anal Calorim. 2019;138:2955–67. https://doi.org/10.1007/s10973-019-08784-7.
Nocuń-Wczelik W, Stolarska K. Calorimetry in the studies of by-pass cement kiln dust as an additive to the calcium aluminate cement. J Therm Anal Calorim. 2019;138:4561–9. https://doi.org/10.1007/s10973-019-08913-2.
Wilińska I, Pacewska B. Comparative investigation of reactivity of different kinds of fly ash in alkaline media. J Therm Anal Calorim. 2019;138:3857–72. https://doi.org/10.1007/s10973-019-08296-4.
Langaro EA, Costa de Moraes M, Buth IS, et al. Use of slag (GBFS) generated in charcoal blast furnace as raw material in alkali-activated cement. J Therm Anal Calorim. 2020;142:1223–31. https://doi.org/10.1007/s10973-020-09632-9.
Robbie AM. Research, CICERO Center for International Climate. Global 2 emissions from cement production. 2017. J Earth Syst Sci Data. https://doi.org/10.5194/essd-10-195-2018.
Scrivener KL, Vanderley JM, Gartner EM. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem Concr Res. 2018;114:2–26.
OECD/IEA, CSI, Low-carbon transition in the cement industry: technology roadmap, International Energy Agency, IEA, Paris, 2018. https://webstore.iea.org/technology-roadmap-low-carbontransition-in-the-cement-industry. Accessed 30 Nov 2019.
Ruppert J, Claude L. CEMBUREAU cement CO2 emission share. Brussels: Task Force Low Carbon Economy; 2017.
Le Quéré C, Global carbon budget, et al. Earth Syst. Sci Data. 2018;10(2018):2141–94. https://doi.org/10.5194/essd-10-2141-2018.
Schneider M. The cement industry on the way to a low-carbon future. Cem Concr Res. 2019;124:105792.
Yang KH, Jung YB. Cho MS, Tae SH Effect of supplementary cementitious materials on reduction of CO2 emissions from concrete. J Clean Prod. 2015;103:774–83.
Juenger MCG, Siddique R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem Concr Res. 2015;78:71–80.
Scrivener KL, John VM, Gartner EM. Eco efficient cements, United Nations Environment Program Sustainable Building and Climate Initiative (UNEP-SBCI) (2016).
CSI Global Cement Data base on CO2 and Energy Information,GNR, Getting the Numbers Right Database, Clinker to Cementitious Ratios: Weighted Average: EU Countries 96% Coverage, World 19% Coverage in 2016. https://www.wbcsdcement.org/GNR-2016/world/GNR-Indicator_59cAWcm-world.html, CEMBUREAU, Domestic Deliveries of Cement Types and Strength: Cembureau Countries - Synthesis (2016).
Deja J, Konieczny D, Krechowiecki G, Pilch Z, Środa B. Cement i beton w gospodarce niskoemisyjnej (Cement and concrete in low-emission economy), Polish Cement Association conference in Kraków, 1.10. 2019 (in Polish).
Polish Cement Association annual reports 2008–2017.
The cement industry in Poland. Important role of the cement sector in the EU economy, Brussels 2019–11–08 .
Scrivener KL. Eco-effcient cements: potential, economically viable solutions for a low-CO2. Paris: Cement-based Materials Industry; 2016.
Lothenbach B, Scrivener KL, Hooton RD. Supplementary cementitious materials. Cem Concr Res. 2011;41:1244–56.
Juenger MCG, Snellings R, Bernal SA. Supplementary cementitious materials: new sources, characterization, and performance insight. Cem Concr Res. 2019;122:257–73.
Pacewska B, Wilińska I. Usage of supplementary cementitious materials: advantages and limitations. J Therm Anal Calorim. 2020;142:371–93. https://doi.org/10.1007/s10973-020-09907-1.
Yang K-H, Jung Y-B, Cho M-S, Tae S-H. Effect of supplementary cementitious materials on reduction of CO2 emissions from concrete. J Clean Prod. 2015;103:774–83.
Bruin M, De Vries P Slag cements: green, strong and cool!. In: 5th International Slag Valorisation Symposium, Leuven, 03–05/04/2017. 239–242.
Ehrenberg, A., Feldrappe, V. Potentials of new cements made from granulated blast furnace slag, fly ash and clinker. In: 6th international slag valorisation symposium, Mechelen, 05.04.2019, Belgium, 97–100.
Durdziński PT, Ben Haha M, Zając M, Scrivener KL. Phase assemblage of composite cements. Cem Concr Res. 2017;99:172–82.
Hooton RD, The reactivity and reaction products of blast-furnace slag. In: Malhotra VM (ed), Supplementary cementing materials for concrete, Ottawa, Canada, 1987; 245–288.
Malhotra VM. Properties of fresh and hardened concrete incorporating ground, granulated blast furnace slag. In: Malhotra VM editors, Supplementary cementing materials for concrete, Ottawa, Canada, 1987; 289-–33.
Sideris K, Justnes HM, Soutsos,M, Sui T. Fly ash, In: De Belie N, Soutsos M, Gruyaert E editors, Prop. fresh hardened concr. contain. suppl. cem. mater. state-of-the-art rep. RILEM Tech. Comm. 238-SCM, Work. Gr. 4, Springer International Publishing, Cham, 2018, pp. 55–98. https://doi.org/10.1007/978-3-319-70606-1_2.
Matthes W, Vollpracht A, Villagrán Y, Kamali-Bernard S, Hooton D, Gruyaert,E, Soutsos M, De Belie N. Ground granulated blast-furnace slag, RILEM state-of-the-art reports, 2018, pp. 1–53. https://doi.org/10.1007/978-3-319-70606-1_1.
Skibsted J, Snellings R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem Concr Res 2019;124:105799.
Giergiczny Z, Szybilski M. Nowelizacja normy EN 197–1 – Trójskładnikowe cementy powszechnego użytku o niskiej zawartości klinkieru portlandzkiego (EN Amendment to standard EN 197–1 – common ternary cements with low Portland clinker content). Mater Bud. 2014;11:3–5 (in Polish).
Edwards P. SCM supplies under pressure from rising demand, Glob Cem Mag 2018;19–21.
Apex S. Ferrous slag trends 2027, Glob Cem Mag 2018;211–26.
Battagin AF, Pecchio M. Blast furnace slag weathering study. In: Proc. 11th Int. Congress on the Chemistry of Cement, Durban 11–16.05.2003;905–912.
Hiroshima A, Igarashi T. Effect by weathering of granulated blast furnace slag powder on the quality of portland blast furnace slag cement, CAJ-Rev 37th General Meet 1983;65–66.
Demoulian E, Vernet C, Hawthorn F, Gourdin P. Détermination de la teneur en laitier dans le ciments par dissolucion seletive. In. Proc. 7th Int. Congr. on the Chemistry of Cement, Paris, 1980, vII, 151–156.
Demoulian E, Gourdin P, Hawthorn F, Vernet C. Influence de la composition chimique et de la texture des laitiers sur leur hydraulicité. In: 7th Int. Congress on the Chemistry of Cement, Paris, 1980, v4, 17–20.
Matschei T, Lothenbach B, Glasser FP. The role of calcium carbonate in cement hydration. Cem Concr Res. 2007;37(4):551–8.
Pacierpnik W, Nocuń-Wczelik W, Kapeluszna E. Application of granulated blast furnace slag from the old storage yards as supplementary cementing materials. Arch Civ Eng. 2020 (in press).
Lothenbach B, Le Saout G, Gallucci E, Scrivener KL. Influence of limestone on the hydration of Portland cements. Cem Concr Res. 2008;38(6):848–60.
Sato T, Beaudoin J. Effect of nano-CaCO3 on hydration of cement containing supplementary cementitious materials. Adv Cem Res. 2011;23(1):33–43.
Weerdt K, Ben Haha M, Le Saout G, Kjellsen KO, Justnes H, Lothenbach B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem Concr Res. 2011;41:279–91.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nocuń-Wczelik, W., Pacierpnik, W. & Kapeluszna, E. Application of calorimetry and other thermal methods in the studies of granulated blast furnace slag from the old storage yards as supplementary cementitious material. J Therm Anal Calorim 147, 8157–8168 (2022). https://doi.org/10.1007/s10973-021-11161-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11161-y