Abstract
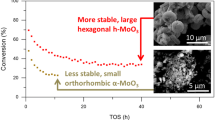
Mechanochemical method has applied to the green preparation of iron-molybdenum catalyst efficiently, and their catalytic performance was evaluated by the oxidation of methanol to formaldehyde. In order to investigate the formation process of iron-molybdenum catalyst based on mechanochemical method, various characterization techniques have been employed. Results indicate that iron-molybdenum catalyst could not be generated during ball milling process without calcining, and calcination is crucial step to regulate the ratio of MoO3 and Fe2(MoO4)3. For the formation of MoO3 and Fe2(MoO4)3 phase, 180 °C could be the key turning temperature point. Fe2(MoO4)3 and MoO3 phases are concurrently emerged when Mo/Fe atomic ratio exceeds 1.5. The aggregation of Fe2(MoO4)3 is severe with the increasing calcination temperature. Fe2(MoO4)3 is stable below 600 °C, while MoO3 phase could be subliming with the increasing temperature. The catalytic performance of iron-molybdenum catalyst has closely correlation with the phase compositions, which can be controlled by synthesis temperature and Mo/Fe molar ratio. The iron-molybdenum catalyst with Mo/Fe atomic ratio of 2.6 calcined at 500 °C for 4 h showed the best methanol conversion (100%) and formaldehyde yield (92.27%).











Similar content being viewed by others
References
Sprenger P, Stehle M, Gaur A, Gänzler AM, Gashnikova D, Kleist W, et al. Reactivity of bismuth molybdates for selective oxidation of propylene probed by correlative operando spectroscopies. ACS Catal. 2018;8(7):6462–75.
Schuh K, Kleist W, Høj M, Trouillet V, Beato P, Jensen AD, et al. Selective oxidation of propylene to acrolein by hydrothermally synthesized bismuth molybdates. Appl Catal A Gen. 2014;482:145–56.
Peng HY, Liu QH, Zhang JQ, Zhang Y, Geng MJ, Yu JQ, et al. Enhanced visible-light-driven photocatalytic activity by 0D/2D Phase heterojunction of quantum dots/nanosheets on bismuth molybdates. J Phys Chem C. 2018;122(7):3738–47.
Chen DM, Hao Q, Wang Z, Ding H, Zhu YF. Influence of phase structure and morphology on the photocatalytic activity of bismuth molybdates. CrystEngComm. 2016;18:1976–86.
Chen HCH, Chen S, Zhu YY, Li CH, Fan MQ, Chen D, Tian GL, et al. Synergistic effect of Ni and Co ions on molybdates for superior electrochemical performance. Electrochim Acta. 2016;190:57–63.
Karthik R, Kumar JV, Chen S, Karuppiah C, Cheng YH, Muthuraj V. A study of electrocatalytic and photocatalytic activity of cerium molybdate nanocubes decorated graphene oxide for the sensing and degradation of antibiotic drug chloramphenicol. ACS Appl Mater Interfaces. 2017;9:6547–59.
Peng CH, Gao L, Yang SW, Sun J. A general precipitation strategy for large-scale synthesis of molybdate nanostructures. Chem Commun. 2008;43:5601–3.
Ju ZhCh, Zhang E, Zhao YL, Xing Z, Zhuang QCh, Qiang YH, et al. One-pot hydrothermal synthesis of FeMoO4 nanocubes as an anode material for lithium-ion batteries with excellent electrochemical performance. Small. 2015;11(36):4753–61.
Wang JX, Zhang GB, Liu Z, Li HK, Liu Y, Wang Z, et al. Li3V(MoO4)3 as a novel electrode material with good lithium storage properties and improved initial coulombic efficiency. Nano Energy. 2018;44:272–8.
Brookes C, Bowker M, Wells PP. Catalysts for the selective oxidation of methanol. Catalysis. 2016;92:1–27.
Soares APV, Portela MF, Kiennemann A. Methanol selective oxidation to formaldehyde over iron-molybdate. Catal Rev. 2005;47:125–74.
Adkins H, Peterson WR. The oxidation of methanol with air over iron, molybdenum, and iron-molybdenum oxides. J Am Chem Soc. 1931;53:1512–20.
Raun KV, Lundegaard LF, Chevallier J, Beato P, Appel CC, Nielsen K, et al. Deactivation behavior of an iron-molybdate catalyst during selective oxidation of methanol to formaldehyde. Catal Sci Technol. 2018;8:4626–37.
House MP, Carley AF, Bowker M. Selective oxidation of methanol on iron molybdate catalysts and the effects of surface reduction. J Catal. 2007;252:88–96.
Routray K, Zhou W, Kiely CJ, Grünert W, Wachs IE. Origin of the synergistic interaction between MoO3 and iron molybdate for the selective oxidation of methanol to formaldehyde. J Catal. 2010;275:84–98.
Jin GJ, Weng WH, Lin Z, Dummer NF, Taylor SH, Kiely CJ, et al. Fe2(MoO4)3/MoO3 nano-structured catalysts for the oxidation of methanol to formaldehyde. J Catal. 2012;296:55–64.
Heim LE, Konnerth H, Prechtl MHG. Future perspectives for formaldehyde: pathways for reductive synthesis and energy storage. Green Chem. 2017;19:2347–55.
Bahmanpour AM, Hoadley A, Mushrif SH, Tanksale A. Hydrogenation of carbon monoxide into formaldehyde in liquid media. ACS Sustain Chem Eng. 2016;4:3970–7.
Choksi T, Greeley J. Partial oxidation of methanol on MoO3 (010): a DFT and microkinetic study. ACS Catal. 2016;6(11):7260–77.
Gribovskii AG, Ovchinnikova EV, Vernikovskaya NV, Andreev DV, Chumachenko VA, Makarshin LL. Microchannel reactor for intensifying oxidation of methanol to formaldehyde over Fe–Mo catalyst. Chem Eng J. 2017;308:135–41.
Yeo BR, Pudge GJF, Bugler KG, Rushby AV, Kondrat S, Bartley J, et al. The surface of iron molybdate catalysts used for the selective oxidation of methanol. Surf Sci. 2016;648:163–9.
Hicham OH. Synthesis, characterization and catalytic performance of iron molybdate Fe2(MoO4)3 nanoparticles. Catal Commun. 2015;60:19–22.
Alia TT, Basahel SN, Mahmoud HA, Khalil KMS, Narasimharao K. Influence of preparation conditions on the catalytic activity of high surface area silica in partial methanol oxidation. Chem Eng J. 2017;330:852–62.
House MP, Carley AF, Echeverria-Valda R, Bowker M. Effect of varying the cation ratio within iron molybdate catalysts for the selective oxidation of methanol. J Phys Chem C. 2008;112(11):4333–41.
Beale AM, Jacques SDM, Sacaliuc-Parvalescu E, O’Brien MG, Barnes P, Weckhuysen BM. An iron molybdate catalyst for methanol to formaldehyde conversion prepared by a hydrothermal method and its characterization. Appl Catal A Gen. 2009;363(1–2):143–52.
Soares APV, Portela MF, Kiennemann A, Hilaire L. Mechanism of deactivation of iron-molybdate catalysts prepared by coprecipitation and sol-gel techniques in methanol to formaldehyde oxidation. Chem Eng Sci. 2003;58(7):1315–22.
Ebert DY, Dorofeeva NV, Saveleva AS, Kharlamov TS, Salaev MA, Svetlichnyi VA, et al. Silica-supported Fe–Mo–O catalysts for selective oxidation of propylene glycol. Catal Today. 2019;333:133–9.
Dias APS, Rozanov VV, Waerenborgh JCB, Portela MF. New Mo–Fe–O silica supported catalysts for methanol to formaldehyde oxidation. Appl Catal A Gen. 2008;345:185–94.
Huang Y, Cong LY, Yu J, Eloy P, Ruiz P. The surface evolution of a catalyst jointly influenced by thermal spreading and solid-state reaction: a case study with an Fe2O3–MoO3 system. J Mol Catal A: Chem. 2009;302(1–2):48–53.
Klissurski D, Mancheva M, Iordanova R, Tyuliev G, Kunev B. Mechanochemical synthesis of nanocrystalline nickel molybdates. J Alloys Compd. 2006;422:53–7.
Skwarek E, Khalameida S, Janusz W, Sydorchuk V, Konovalova N, Zazhigalov V, et al. Influence of mechanochemical activation on structure and some properties of mixed vanadium-molybdenum oxides. J Therm Anal Calorim. 2011;106:881–94.
Sydorchuk V, Makota O, Khalameida S, Bulgakova L, Skubiszewska-Zieba J, Leboda R, et al. Physical-chemical and catalytic properties of deposited MoO3 and V2O5. J Therm Anal Calorim. 2012;108:1001–8.
Radev DD, Blaskov V, Klissurski D, Mitov I, Toneva A. Effect of the mechanical activation of the reagents on the solid phase synthesis of iron (III) molybdate. J Alloys Compd. 1997;256:108–11.
James SL, Adams CJ, Bolm C, Braga D, Collier P, Friščić T, et al. Mechanochemistry: opportunities for new and cleaner synthesis. Chem Soc Rev. 2012;41:413–47.
Do JK, Friščić T. Mechanochemistry: a force of synthesis. ACS Cent Sci. 2017;3:13–9.
Muñoz-Batista MJ, Rodriguez-Padron D, Puente-Santiago DAR, Luque R. Mechanochemistry: toward sustainable design of advanced nanomaterials for electrochemical energy storage and catalytic applications. ACS Sustain Chem Eng. 2018;6(8):9530–44.
Michalchuk AAL, Tumanov IA, Boldyreva EV. Ball size or ball mass what matters in organic mechanochemical synthesis? CrystEngComm. 2019;21:2174–9.
Huang Y, Ruiz P. The nature of antimony-enriched surface layer of Fe–Sb mixed oxides. Appl Surf Sci. 2006;252(22):7849–55.
Szymanski MP, Jedrzejewska H, Wierzbicki M, Szumna A. On the mechanism of mechanochemical molecular encapsulation in peptidic capsules. Phys Chem Chem Phys. 2017;19:15676.
Wu Q, Liu X, Zhu L, Ding L, Gao P, Wang X, et al. Solvent-free synthesis of zeolites from anhydrous starting raw solids. J Am Chem Soc. 2015;137(3):1052–5.
Kong LT, Zhang M, Liu X, Ma FY, Wei B, Wumaier K, et al. Green and rapid synthesis of iron molybdate catalyst by mechanochemistry and their catalytic performance for the oxidation of methanol to formaldehyde. Chem Eng J. 2019;364:390–400.
Ivanov K, Dimitrov D, Boyanov B. Optimization of the methanol oxidation over iron-molybdate catalysts. Chem Eng J. 2009;154:189–95.
Said AEA, El-Wahab MMMA, Alian AM. Selective oxidation of methanol to formaldehyde over active molybdenum oxide supported on hydroxyapatite catalysts. Catal Lett. 2016;146:82–90.
Dias APS, Montemor F, Portela MF, Kiennemann A. The role of the suprastoichiometric molybdenum during methanol to formaldehyde oxidation over Mo–Fe mixed oxides. J Mol Catal A: Chem. 2015;397:93–8.
Wang L, Zhang GH, Sun YJ, Zhou XE, Chou KC. Preparation of ultrafine β-MoO3 from industrial grade MoO3 powder by the method of sublimation. J Phys Chem C. 2016;120:19821–9.
Mizushima T, Moriya Y, Phuc NHH, Ohkita H, Kakuta N. Soft chemical transformation of α-MoO3 to β-MoO3 as a catalyst for vapor-phase oxidation of methanol. Catal Commun. 2011;13:10–3.
Zhang S, Zhang YK, Hu R, Zhen B, Han MH. Surface structure and activity of iron molybdate catalyst for methanol oxidation to formaldehyde. CIESC J. 2016;67(9):3678–83.
Sum-Kou MR, Mendioroz S, Fierro JLG, Palacios JM. Influence of the preparation method on the behaviour of Fe–Mo catalysts for the oxidation of methanol. J Mater Sci. 1995;30(2):496–503.
Li W, Liu HCh, Iglesia E. Structures and properties of zirconia-supported ruthenium oxide catalysts for the selective oxidation of methanol to methyl formate. J Phys Chem B. 2006;110(46):23337–42.
Li D, Xue JQ, Ma J, Tang JL. Synthesis of Fe2(MoO4)3/MoO3 heterostructured microrods and photocatalytic performances. New J Chem. 2016;40:3330–5.
Xu Q, Jia GQ, Zhang J, Feng ZhCh, Li C. Surface phase composition of iron molybdate catalysts studied by UV Raman spectroscopy. J Phys Chem C. 2008;112(25):9387–93.
Hill CG, Wilson JH. Raman spectroscopy of iron molybdate catalyst systems: part I. Preparation of unsupported catalysts. J Mol Catal. 1990;63(1):65–94.
Soares APV, Portela MF, Kiennemann A. Iron molybdates for selective oxidation of methanol: Mo excess effects on the deactivation behaviour. Catal Commun. 2001;2(5):159–64.
Andersson A, Hernelind M, Augustsson O. A study of the ageing and deactivation phenomena occurring during operation of an iron molybdate catalyst in formaldehyde production. Catal Today. 2006;112(1–4):40–4.
Ivanov KI, Dimitrov DY. Deactivation of an industrial iron-molybdate catalyst for methanol oxidation. Catal Today. 2010;154(3–4):250–5.
Bowker M, House M, Alshehri A, Brookes C, Gibson EK, Wells PP. Selectivity determinants for dual function catalysts: applied to methanol selective oxidation on iron molybdate. Catal Struct React. 2015;1(2):95–100.
Bowker M, Gibson EK, Silverwoodad IP, Brookes C. Methanol oxidation on Fe2O3 catalysts and the effects of surface Mo. Faraday Discuss. 2016;188:387–98.
Acknowledgements
We thank the financial supports from Natural Science Foundation Project of Xinjiang Uygur Autonomous (2019D01C084). We thank Xinjiang University Institute of experimental center for material characterization and analysis. We also thank State Key Laboratory of Chemical Engineering at East China University of Science and Technology in where we were provided the chance for in situ XRD analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, X., Kong, Lt., Liu, Cf. et al. Study on the formation process of MoO3/Fe2(MoO4)3 by mechanochemical synthesis and their catalytic performance in methanol to formaldehyde. J Therm Anal Calorim 142, 1363–1376 (2020). https://doi.org/10.1007/s10973-020-09483-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09483-4