Abstract
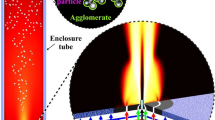
This study mathematically presents a counterflow non-premixed thermochemical technique for preparing a particle oxide used for cancer diagnosis and treatment. For this purpose, preheating, reaction, melting, and oxidation processes were simulated considering an asymptotic concept. Mass and energy conservation equations in dimensional and non-dimensional forms were solved using MATLAB®. To preserve the continuity in the system and calculate the locations of melting and flame fronts, promising jump conditions were derived. In this research, variations in flame temperature, flame front location and mass fractions of the particle, particle oxide and oxidizer, with position, Lewis number and initial temperature of the particles were investigated. The simulation results were compared with those obtained from an earlier experimental study under the same conditions. Regarding the comparison, an appropriate compatibility was observed between the results. Based on the simulation results, flame temperature was found to be about 1310 K. Positions of flame and melting fronts were found to be − 1.8 mm and − 1.78 mm, respectively.







Similar content being viewed by others
Abbreviations
- a :
-
Strain rate \(\left( {\frac{1}{a}} \right)\)
- C :
-
Mixture specific heat capacity (kJ kg−1 K−1)
- C a :
-
Heat capacity of the gas (kJ kg−1 K−1)
- C p :
-
Heat capacity of the particle (kJ kg−1 K−1)
- D C :
-
Damkohler number
- D F :
-
Diffusion coefficient of particle (m2 s−1)
- D m :
-
Diffusion coefficient of particle oxide in liquid phase (m2 s−1)
- D O :
-
Diffusion coefficient of oxidizer (m2 s−1)
- D T :
-
Thermal diffusion coefficient (m2 s−1)
- E :
-
Overall activation energy (kJ)
- erf(x):
-
Error function
- H :
-
Heaviside function
- Le:
-
Lewis number
- m :
-
Mixture molecular mass (kg mol−1)
- m F :
-
Fuel molecular mass (kg mol−1)
- m O :
-
Oxidizer molecular mass (kg mol−1)
- n p :
-
Local number density of particles (number of particles per unit volume)
- \({\mathcal{Q}}\) :
-
Heat of reaction (kJ kg−1)
- Q melt :
-
Latent heat of melting (kJ kg−1)
- \({\fancyscript{q}}_{\rm melt}\) :
-
Ratio of latent heat of melting to the heat released from reaction
- R :
-
The universal gas constant (m3 Pa mol−1 K−1)
- r p :
-
Particle radius (µm)
- T :
-
Temperature (K)
- T a :
-
Activation temperature (K)
- T ad :
-
Adiabatic temperature (K)
- T f :
-
Flame temperature (K)
- T ig :
-
Ignition temperature of particles (K)
- T melt :
-
Melting temperature of particle oxide (K)
- T ∞ :
-
Ambient temperature (K)
- \({\mathcal{W}}_{\text{F}}\) :
-
Molecular weight of the particle (kg kmol−1)
- x :
-
Dimensional length (m)
- x f :
-
Dimensional flame sheet position (m)
- X f :
-
Non-dimensional flame sheet position
- x melt :
-
Onset position of melting in dimensional form (m)
- X melt :
-
Onset position of melting in non-dimensional form
- Y m :
-
Mass fraction of particle oxide in liquid phase
- Y O :
-
Mass fraction of the oxidizer
- Y S :
-
Mass fraction of the particle
- Y S−∞ :
-
Mass fraction of the particle at the distance − ∞
- y m :
-
Dimensionless form of the mass fraction of particle oxide in liquid phase
- y O :
-
Dimensionless form of the mass fraction of oxidizer
- y S :
-
Dimensionless form of the mass fraction of particle
- ω melt :
-
Melting rate of particle oxide (kg m−1 s−2)
- \(\widetilde{\omega }_{\text{melt}}\) :
-
Dimensionless form of melting
- ω S :
-
Reaction rate (kg m−1 s−2)
- \(\widetilde{\omega }_{\text{S}}\) :
-
Dimensionless form of the reaction rate
- τ melt :
-
Constant characteristic time of melting
- λ :
-
Thermal conductivity (kJ m−1 s−1 K)
- ρ :
-
Density of the mixture (kg m−3)
- ρ a :
-
Density of the gas (kg m−3)
- ρ p :
-
Density of the particle (kg m−3)
- θ :
-
Dimensionless form of the temperature
- θ f :
-
Dimensionless form of the flame temperature
- θ melt :
-
Dimensionless form of the melting temperature
- v O :
-
Oxidizer stoichiometric coefficient
- v p :
-
Product stoichiometric coefficient
- v S :
-
Fuel stoichiometric coefficient
- a:
-
Gas
- f:
-
Flame
- melt:
-
Melting
- P:
-
Product
- S:
-
Particle
- V:
-
Velocity
- ∞:
-
Ambient condition
References
Shahbazi-Gahrouei D, Keshtkar M. Magnetic nanoparticles and cancer treatment. Immunopathol Persa. 2016;2(1):e03.
Berry CC. Progress in functionalization of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2009;42(22):224003.
Atiq S, Ansar MZ, Riaz S, Naseem S. Synthesis and characterization of magnetic nanoparticles for cancer therapy. In: Proceedings of 2013 world congress on advances in nano, biomechanics, robotics and energy research (ANBRE), 25 Aug 2013.
Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Future Med. 2007;2:23–39.
Wu W, Wu Z, Yu T, Jiang C, Kim WS. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater. 2015;16(2):023501.
Arruebo M, Fernández-Pacheco R, Ibarra MR, Santamaría J. Magnetic nanoparticles for drug delivery. Nano Today. 2007;2(3):22–32.
Moacă EA, Coricovac ED, Soica CM, Pinzaru IA, Păcurariu CS, Dehelean CA. Preclinical aspects on magnetic iron oxide nanoparticles and their interventions as anticancer agents: enucleation, apoptosis and other mechanism. In: Shatokha V, editor. Iron ores and iron oxide materials. London: IntechOpen; 2018. p. 229.
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108(6):2064–110.
Mata-Pérez F, Martínez JR, Guerrero AL, Ortega-Zarzosa G. New way to produce magnetite nanoparticles at low temperature. Adv Chem Eng Res. 2015;4(1):48–55. https://doi.org/10.12783/acer.2015.0401.04.
Guo B, Kennedy IM. Gas-phase flame synthesis and characterization of iron oxide nanoparticles for use in a health effects study. Aerosol Sci Technol. 2007;41(10):944–51.
Ali A, Hira Zafar MZ, ul Haq I, Phull AR, Ali JS, Hussain A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl. 2016;9:49.
Ling D, Hyeon T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small. 2013;9(9-10):1450–66.
Stumm W, Lee GF. Oxygenation of ferrous iron. Ind Eng Chem. 1961;53(2):143–6.
McAllister S, Chen JY, Fernandez-Pello AC. Fundamentals of combustion processes. New York: Springer; 2011.
Shadloo MS. Numerical simulation of compressible flows by lattice Boltzmann method. Numer Heat Transf Part A Appl. 2019;75(3):167–82.
Hopp-Hirschler M, Shadloo MS, Nieken U. Viscous fingering phenomena in the early stage of polymer membrane formation. J Fluid Mech. 2019;864:97–140.
Shadloo MS, Poultangari R, Jamalabadi MA, Rashidi MM. A new and efficient mechanism for spark ignition engines. Energy Convers Manag. 2015;96:418–29.
Hopp-Hirschler M, Shadloo MS, Nieken U. A smoothed particle hydrodynamics approach for thermo-capillary flows. Comput Fluids. 2018;176:1–19.
Shadloo MS, Hadjadj A. Laminar-turbulent transition in supersonic boundary layers with surface heat transfer: a numerical study. Numer Heat Transf Part A Appl. 2017;72(1):40–53.
Daoush WM. Co-precipitation and magnetic properties of magnetite nanoparticles for potential biomedical applications. J Nanomed Res. 2017;5(1):e6.
Andrade ÂL, Valente MA, Ferreira JM, Fabris JD. Preparation of size-controlled nanoparticles of magnetite. J Magn Magn Mater. 2012;324(10):1753–7.
Setyawan H, Widiyastuti W. Progress in the preparation of magnetite nanoparticles through the electrochemical method. KONA Powder Particle J. 2019;36:145–55.
Dresco PA, Zaitsev VS, Gambino RJ, Chu B. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir. 1999;15(6):1945–51.
Koushika EM, Shanmugavelayutham G, Saravanan P, Balasubramanian C. Rapid synthesis of nano-magnetite by thermal plasma route and its magnetic properties. Mater Manuf Process. 2018;33(15):1701–7.
Rashid H, Mansoor MA, Haider B, Nasir R, Abd Hamid SB, Abdulrahman A. Synthesis and characterization of magnetite nano particles with high selectivity using in situ precipitation method. Sep Sci Technol. 2019;2:1–9.
de Almeida Silva R, Castro CD, Vigânico EM, Petter CO, Schneider IA. Selective precipitation/UV production of magnetite particles obtained from the iron recovered from acid mine drainage. Min Eng. 2012;29:22–7.
Lei P, Girshick SL. Thermal plasma synthesis of superparamagnetic iron oxide nanoparticles for biomedical applications. Plasma Chem Plasma Process. 2012. https://doi.org/10.1007/s11090-012-9364-1.
Owens GI, Singh RK, Foroutan F, Alqaysi M, Han CM, Mahapatra C, Kim HW, Knowles JC. Sol–gel based materials for biomedical applications. Prog Mater Sci. 2016;77:1–79.
Bidabadi M, Panahifar P, Sadeghi S. Analytical development of a model for counter-flow non-premixed flames with volatile biofuel particles considering drying and vaporization zones with finite thicknesses. Fuel. 2018;231:172–86.
Nematollahi M, Rasam H, Sadeghi S, Bidabadi M. Asymptotic prediction of multi-region planar non-premixed combustion of moisty porous coal particles in counter-flow design considering pyrolysis, homogeneous and heterogeneous reactions. Combust Flame. 2019;207:281–94.
Bidabadi M, Ramezanpour M, Poorfar AK, Monteiro E, Rouboa A. Mathematical modeling of a non-premixed organic dust flame in a counterflow configuration. Energy Fuels. 2016;30(11):9772–82.
Sun JH, Dobashi R, Hirano T. Temperature profile across the combustion zone propagating through an iron particle cloud. J Loss Prev Process Ind. 2001;14(6):463–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tabaei, A., Sadeghi, S., Hosseinzadeh, S. et al. A simplified mathematical study of thermochemical preparation of particle oxide under counterflow configuration for use in biomedical applications. J Therm Anal Calorim 139, 2769–2779 (2020). https://doi.org/10.1007/s10973-019-08917-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08917-y