Abstract
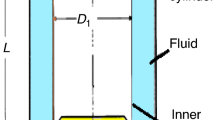
In mixing hazard evaluations, a smaller scale is preferred for safety reasons and in order to conserve the samples. We have developed a small-scale Dewar vessel test (SDVT) that measures the amount of heat from a temperature change upon the mixing of two chemicals. In general, the accuracy of small-scale tests is negatively impacted by heat loss. The purpose of this study is to establish experimental conditions and to validate the reproducibility and accuracy of our small-scale test. The adjustable experimental parameters for SDVT are: position of sample injection, amount of sample, stirring speed, and surrounding temperature. These conditions were optimized for the measurement of the heat of neutralization of hydrochloric acid and sodium hydroxide. Under the optimized experimental conditions, the reproducibility was validated by measurement of the heat of hydration between acetic acid anhydride and water. The relative standard deviation of the maximum temperature change was 3.1%. To validate the accuracy, the heat of reaction between the neutralization reaction and hydration reaction was calculated. The heat of reaction was in good agreement with the theoretical value. Thus, the SDVT has sufficient accuracy and reproducibility to serve as a screening method for the mixing hazard of chemicals.










Similar content being viewed by others
References
Miyake A, Yamada N, Ogawa T. Mixing hazard evaluation of organic peroxides with other chemicals. J Loss Prev Process Ind. 2005;18:380–3.
Miyake A, Kimura A, Yamada N. Investigation of an accidental explosion due to unintended mixing involving reactive chemicals at a waste storage tank. J Mater Cycles Waste Manag. 2008;10:124–8.
Fujita M, Iizuka Y, Miyake A. Thermal and kinetic analyses on Michael addition reaction of acrylic acid. J Therm Anal Calorim. 2017;128:1227–33.
Yamamoto Y, Miyake A. Influence of a mixed solvent containing ionic liquids on the thermal hazard of the cellulose dissolution process. J Therm Anal Calorim. 2017;127:743–8.
Babasaki Y, Iizuka Y, Miyake A. Influence of organic acid on the thermal behavior of dimethyl sulfoxide. J Therm Anal Calorim. 2015;121:295–301.
Fujimoto Y. Methods for evaluating reaction hazards. Specific research reports of the National Institute of Occupational Safety and Health, vol. 34. 2006. pp. 5–10.
Ando T, Fujimoto Y, Kumasaki M. A survey on evaluation techniques of reaction runaway hazards in chemical processes. Specific research reports of the National Institute of Occupational Safety and Health, vol. 27. 2002. pp. 5–10.
Miyake A, Kimura A, Satoh Y, Shimizu R, Inano M, Ogawa T. Thermal hazard analysis of mixed system of hydrazine and nitric acid. J Therm Anal Calorim. 2004;85:633–6.
Miyake A, Kimura A, Ogawa T, Satoh Y, Inano M. Thermal hazard analysis of hydrazine and nitric acid mixtures. J Therm Anal Calorim. 2005;80:515–8.
Talouba I, Balland L, Bensahla N, Mouhab N. Thermokinetic parameter determination of methacrylates radical polymerization by using real-time reaction calorimetry. J Therm Anal Calorim. 2017;130:2341–9.
Zang N, Qian X, Liu Z, Shu C. Thermal hazard evaluation of cyclohexanone peroxide synthesis. J Therm Anal Calorim. 2016;124:1131–9.
Chen C, Wu C. Thermal hazard assessment and macrokinetics analysis of toluene mononitration in a batch reactor. J Loss Prev Process Ind. 1996;9:309–16.
Visentin F, Gianoli S, Zogg A, Kut O, Hungerbühler K. A pressure-resistant small-scale reaction calorimeter that combines the principles of power compensation and heat balance (CRC. v4). Org Process Res Dev. 2004;8:725–37.
Singh J. Reaction calorimetry for process development: recent advances. Process Saf Prog. 1997;16:43–9.
United Nations. Recommendations on the transport of dangerous goods. Manual of tests and criteria, 5th revised edition. New York: United Nations; 2009.
Fierz H. Influence of heat transport mechanisms on transport classification by SADT-measurement as measured by the Dewar-method. J Hazard Mater. 2003;96:121–6.
Li X, Koseki H. Study on the early stage of runaway reaction using Dewar vessels. J Loss Prev Process Ind. 2005;18:455–9.
Davydenkov I, Milman S, Velikanova M, Kotov L, Perestoronin A. Study of Dewar multishield insulation system at 4.2–293 K. Cryogenics. 1993;33:1137–41.
Fisher H, Goetz D. Determination of self-accelerating decomposition temperatures using the accelerating rate calorimeter. J Loss Prev Process Ind. 1991;4:305–16.
Fisher H, Goetz D. Determination of self-accelerating decomposition temperatures for self-reactive substances. J Loss Prev Process Ind. 1993;6:183–94.
Lu G, Yang T, Chen L, Zhou Y, Chen W. Thermal decomposition kinetics of 2-ethylhexyl nitrate under nonisothermal and isothermal conditions. J Therm Anal Calorim. 2016;124:471–8.
Rao G, Feng W, Zhang J, Wang S, Chen L, Guo Z, Chen W. Simulation approach to decomposition kinetics and thermal hazards of hexamethylenetetramine. J Therm Anal Calorim. 2019;135:2447–56.
Wang B, Yi H, Xu K, Wang Q. Prediction of the self-accelerating decomposition temperature of organic peroxides using QSPR models. J Therm Anal Calorim. 2017;128:399–406.
Yang D, Koseki H, Hasegawa K. Predicting the self-accelerating decomposition temperature (SADT) of organic peroxides based on non-isothermal decomposition behavior. J Loss Prev Process Ind. 2003;16:411–6.
Pastré J, Wörsdörfer U, Keller A, Hungerbühler K. Comparison of different methods for estimating TMRad from dynamic DSC measurements with ADT 24 values obtained from adiabatic Dewar experiments. J Loss Prev Process Ind. 2000;13:7–17.
Sun J, Li X, Hasegawa K, Liao G. Thermal hazard evaluation of complex reactive substance using calorimeters and Dewer vessel. J Therm Anal Calorim. 2004;76:883–93.
Grewer T. Thermal hazards of chemical reactions. Industrial safety series, vol. 4. Amsterdam: Elsevier; 1994.
Tseng J, Lin C. Prediction of incompatible reaction of dibenzoyl peroxide by isothermal calorimetry analysis and green thermal analysis technology. J Therm Anal Calorim. 2012;107:927–33.
Wang Y, Duh Y, Shu C. Evaluation of adiabatic runaway reaction and vent sizing for emergency relief from DSC. J Therm Anal Calorim. 2006;85:225–34.
Kegeles G. The heat of neutralization of sodium hydroxide with hydrochloric acid. J Am Chem Soc. 1940;62:3230–2.
Wadasö I. Heats of aminolysis and hydrolysis of some N-acetyl compounds and of acetic anhydride. Acta Chem Scand. 1962;16:471–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suzuki, R., Izato, Yi., Yoshino, S. et al. Mixing hazard evaluation using small-scale Dewar vessels. J Therm Anal Calorim 140, 835–842 (2020). https://doi.org/10.1007/s10973-019-08861-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08861-x