Abstract
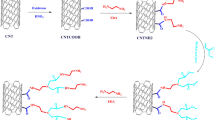
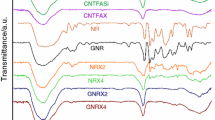
Three different hybrid composites were prepared from tetraethyl orthosilicate oligomer-modified epoxy resin (MER), (3-glycidyloxypropyl)trimethoxysilane-modified novolac resin (MNR), epoxidized novolac resin (ENR), and furfuryl alcohol and 3-(trimethoxysilyl)propyl methacrylate (MPS)-modified carbon nanotubes (CNTS) by combination of sol–gel method and curing reaction. The first hybrid composite (CMEMNH) was prepared from CNTS, MER, and MNR and curing of epoxy groups with ethylenediamine. The second hybrid composite (CENMNH) was prepared from CNTS, ENR, and MNR and subsequent curing of ENR with ethylenediamine. The third hybrid composite (CMENH) was prepared from CNTS and MER and curing of novolac resin with epoxy groups of MER. Finally, the three hybrid composites were thermally evaluated. Successful modification of CNTCOOH by furfuryl alcohol and MPS was confirmed by Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy results. X-ray diffraction patterns for hybrid composites show a broad amorphous peak at diffraction angle of about 19.8° related to silica/siloxane domains. Thermogravimetric analysis results show that the higher char content (54.7) and the most increase in char residue (23.19%) are observed for the CENMNH composite with 4 mass% of CNTS. In the case of CMEMNH and CMENH composites, MER incorporation results in lower char residues. In most of the composites, the presence of CNTS and its incorporation into the hybrid network improve final thermal stability of the product. Scanning electron microscopy and transmission electron microscopy images show tubular structure of CNTS with smooth surface.









Similar content being viewed by others
References
Wen FJ, Wilkes GL. Organic/inorganic hybrid network materials by the sol–gel approach. Chem Mater. 1996;8:1667–81.
Schubert U, Huesing N, Lorenz A. Hybrid inorganic-organic materials by sol–gel processing of organofunctional metal alkoxides. Chem Mater. 1995;7:2010–27.
Park JK, Kang TJ. Thermal and ablative properties of low temperature carbon fiber–phenol formaldehyde resin composites. Carbon. 2002;40:2125–34.
Ma Y, Wang J, Xu Y, Wang C, Chu F. Preparation and characterization of phenolic foams with eco-friendly halogen-free flame retardant. J Therm Anal Claorim. 2013;114:1143–51.
Wu HD, Lee MS, Wu YD, Su YF, Ma CC. Pultruded fiber-reinforced polyurethane-toughened phenolic resin. II. Mechanical properties, thermal properties, and flame resistance. J Appl Polym Sci. 1996;62:227–34.
Dogana M, Unlu SM. Flame retardant effect of boron compounds on red phosphorus containing epoxy resins. Polym Degrad Stabil. 2014;99:12–7.
Wu Q, Bao J, Zhang C, Liang R, Wang B. The effect of thermal stability of carbon nanotubes on the flame retardancy of epoxy and bismaleimide/carbon fiber/buckypaper composites. J Therm Anal Calorim. 2011;103:237–42.
Najafi-Shoa S, Roghani-Mamaqani H, Salami-Kalajahi M. Incorporation of epoxy resin and graphene nanolayers into silica xerogel network: an insight into thermal improvement of resin. J Sol-Gel Sci Technol. 2016;80:362–77.
Han S, Gyu Yoon H, Suh KS, Gun Kim W, Jin Moon T. Cure kinetics of biphenyl epoxy-phenol novolac resin system using triphenylphosphine as catalyst. J Polym Sci A Polym Chem. 1999;37:713–20.
Gualpa MC, Riccardi CC, Vazquez A. Study of the kinetic and crosslinking reaction of novolak with epoxy resin. Polymer. 1998;39:2247–53.
Yadav R, Srivastava D. Blends of cardanol-based epoxidized novolac resin and CTBN for application in surface coating: a study on thermal, mechanical, chemical, and morphological characteristics. J Coat Technol Res. 2010;7:557–68.
Yadav R, Awasthi P, Srivastava D. Studies on synthesis of modified epoxidized novolac resin from renewable resource material for application in surface coating. J Appl Polym Sci. 2009;114:1471–84.
Noparvar-Qarebagh A, Roghani-Mamaqani H, Salami-Kalajahi M. Organic–inorganic nanohybrids of novolac phenolic resin and carbon nanotube: high carbon yields by using carbon nanotube aerogel and resin incorporation into aerogel network. Micropor Mesopor Mater. 2016;224:58–67.
Noparvar-Qarebagh A, Roghani-Mamaqani H, Salami-Kalajahi M. Functionalization of carbon nanotubes by furfuryl alcohol moieties for preparation of novolac phenolic resin composites with high carbon yield values. Colloid Polym Sci. 2015;293:3623–31.
Liu L, Ye Z. Effects of modified multi-walled carbon nanotubes on the curing behavior and thermal stability of boron phenolic resin. Polym Degrad Stabil. 2009;94:1972–8.
Lee SK, Bai BC, Im JS, In SJ, Lee YS. Flame retardant epoxy complex produced by addition of montmorillonite and carbon nanotube. J Ind Eng Chem. 2010;16:891–5.
Kuan CF, Chen WJ, Li YL, Chen CH, Kuan HC, Chiang CL. Flame retardance and thermal stability of carbon nanotube epoxy composite prepared from sol–gel method. J Phys Chem Solids. 2010;71:539–43.
Noparvar-Qarebagh A, Roghani-Mamaqani H, Salami-Kalajahi M. Novolac phenolic resin and graphene aerogel organic–inorganic nanohybrids: high carbon yields by resin modification and its incorporation into aerogel network. Polym Degrad Stabil. 2016;124:1–14.
Wu SY, Yuen SM, Ma CCM, Chiang CL, Huang YL, Wu H, Teng CC, Yang CC, Wei MH. Preparation, morphology, and properties of silane-modified MWCNT/epoxy composites. J Appl Polym Sci. 2010;115:3481–8.
Wu SY, Yuen SM, Ma CCM, Huang YL, Teng CC. Molecular motion, morphology and properties of 3-isocyanato-propyltriethoxysilane-modified multi-walled carbon nanotube/epoxy composites. Micro Nano Lett. 2011;6:463–7.
Najafi-Shoa S, Roghani-Mamaqani H, Salami-Kalajahi M, Azimi R, Gholipour-Mahmoudalilou M. Incorporation of epoxy resin and carbon nanotube into silica/siloxane network for improving thermal properties. J Mater Sci. 2016;51:9057–73.
Wu HL, Yang YT, Ma CCM, Kuan HC. Molecular mobility of free-radical-functionalized carbon-nanotube/siloxane/poly(urea urethane) nanocomposites. J Polym Sci A Polym Chem. 2005;43:6084–94.
Alyamac E, Gua H, Soucek MD, Qiub S, Buchheit RG. Alkoxysilane oligomer modified epoxide primers. Prog Organ Coat. 2012;74:67–81.
Chiang CL, Ma CCM, Wu DL, Kuan HC. Preparation, characterization, and properties of novolac-type phenolic/SiO2 hybrid organic–inorganic nanocomposite materials by sol–gel method. J Polym Sci Part A Polym Chem. 2003;41:905–13.
Cherian AB, Thachil ET. Epoxidized phenolic novolac: a novel modifier for unsaturated polyester resin. J Appl Polym Sci. 2006;100:457–65.
Pan G, Du Z, Zhang C, Li C, Yang X, Li H. Synthesis, characterization, and properties of novel novolac epoxy resin containing naphthalene moiety. Polymer. 2007;48:3686–93.
Tyberg CS, Bergeron K, Sankarapandian M, Shih P, Loos AC, Dillard DA, McGrath JE, Riffle JS, Sorathia U. Structure-property relationships of void-free phenolic–epoxy matrix materials. Polymer. 2000;41:5053–62.
Noparvar-Qarebagh A, Roghani-Mamaqani H, Salami-Kalajahi M, Kariminejad B. Nanohybrids of novolac phenolic resin and carbon nanotube-containing silica network. J Therm Anal Calorim. 2016;2:1027–37.
Roghani-Mamaqani H, Haddadi-Asl V, Mortezaei M, Khezri K. Furfuryl alcohol functionalized graphene nanosheets for synthesis of high carbon yield novolak composites. J Appl Polym Sci. 2014;131:40273.
Men XH, Zhang ZZ, Song HJ, Wang K, Jiang W. Functionalization of carbon nanotubes to improve the tribological properties of poly(furfuryl alcohol) composite coatings. Compos Sci Technol. 2008;68:1042–9.
Gholipour-Mahmoudalilou M, Roghani-Mamaqani H, Azimi R, Abdollahi A. Preparation of hyperbranched poly(amidoamine)-grafted graphene nanolayers as a composite and curing agent for epoxy resin. Appl Surf Sci. 2018;428:1061–9.
Adhikari PD, Jeon S, Cha MJ, Jung DS, Kim Y, Park CY. Immobilization of carbon nanotubes on functionalized graphene film grown by chemical vapor deposition and characterization of the hybrid material. Sci Technol Adv Mater. 2014;15:015007.
Roghani-Mamaqani H, Khezri K. A grafting from approach to graft polystyrene chains to the surface of graphene nanolayers by RAFT polymerization: various graft densities from hydroxyl groups. Appl Surf Sci. 2016;360:373–82.
Azimi R, Roghani-Mamaqani H, Gholipour-Mahmoudalilou M. Grafting poly(amidoamine) dendrimer-modified silica nanoparticles to graphene oxide for preparation of a composite and curing agent for epoxy resin. Polymer. 2017;126:152–61.
Yuen SM, Ma CC, Chiang CL, Teng CC, Yu YH. Poly (vinyltriethoxysilane) modified MWCNT/polyimide nanocomposites-preparation, morphological, mechanical, and electrical properties. J Polym Sci Part A Polym Chem. 2008;46:803–16.
Theodoropoulou S, Papadimitriou D, Zoumpoulakis L, Simitzis J. Structural and optical characterization of pyrolytic carbon derived from novolac resin. Anal Bioanal Chem. 2004;379:788–91.
George GA, Clarke PC, Jhon NS, Friend G. Real time monitoring of the cure reaction of a TGDDM/DDS epoxy resin using fiber optic FT-IR. J Appl Polym Sci. 1991;42:643–57.
Cochet M, Maser WK, Benito AM, Callejas MA, Martinez MT, Benoit JM, Schreiber J, Chauvet O. Synthesis of a new polyaniline/nanotube composite: in situ polymerisation and charge transfer through site-selective interaction. Chem Commun. 2001;1450:1451.
Yao X, Wu H, Wang J, Qu S, Chen G. Carbon nanotube/poly(methyl methacrylate) (CNT/PMMA) composite electrode fabricated by in situ polymerization for microchip capillary electrophoresis. Chem Eur J. 2007;13:846–53.
Lei Z, Yan Y, Feng J, Wu J, Huang G, Li X, Xing W, Zhao L. Enhanced power factor within graphene hybridized carbon aerogels. RSC Adv. 2015;5:25650–6.
Wu C, Huang X, Wang G, Wu X, Yang K, Li S, Jiang P. Hyperbranched-polymer functionalization of graphene sheets for enhanced mechanical and dielectric properties of polyurethane composites. J Mater Chem. 2012;22:7010–9.
Roghani-Mamaqani H, Haddadi-Asl V, Sobhkhiz Z, Ghaderi-Ghahfarrokhi M. Grafting poly(methyl methacrylate) from azo-functionalized graphene nanolayers via reverse atom transfer radical polymerization. Reverse atom transfer radical polymerization of methyl methacrylate in the presence of Azo-functionalized carbon nanotubes: a grafting from approach. Colloid Polym Sci. 2014;292:2971–81.
Bao C, Guo Y, Song L, Kan Y, Qian X, Hu Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J Mater Chem. 2011;21:13290–8.
Acknowledgements
Iran National Science Foundation (INSF) is greatly appreciated for its financial support (Grant No. 95839965).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Ebrahimi, H., Roghani-Mamaqani, H. & Salami-Kalajahi, M. Preparation of carbon nanotube-containing hybrid composites from epoxy, novolac, and epoxidized novolac resins using sol–gel method. J Therm Anal Calorim 132, 513–524 (2018). https://doi.org/10.1007/s10973-018-6992-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6992-6