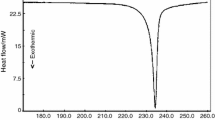
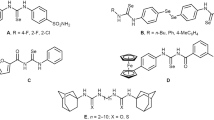
Abstract
The fusion and thermal decomposition of thirty-three diselenide compounds with a urea, thiourea or selenourea group linked with different aliphatic or aromatic substituents have been studied by thermogravimetry, differential scanning calorimetry and mass spectrometry in order to perform comparative thermal stability studies among analogs. A relationship has been found between stability and a series of effects which occur in the compound structures. Analysis of the thermal data indicated that: (a) in general, compounds with a urea or selenourea group are more stable than those with a thiourea group; (b) no difference in stability exists when an aromatic or aliphatic group is linked to the thiourea group but when linked to the urea or selenourea groups, stability does differ; (c) selenourea compounds with aliphatic chain are the most unstable; and (d) the nature of the substituent located on the benzyl ring has no effects on thermal stability. Therefore, criteria for the selection of substituents can be established in order to improve the stability of these drugs. In addition, the mass spectral fragmentation in comparison with thermal analytical data helps in confirming the thermal behavior of the compounds.









Similar content being viewed by others
References
Lever T, Hains P, Rouquerol J, Charsley EL, Eckeren PV, Burlett DJ. ICTAC nomenclature of thermal analysis (IUPAC) recommendations. Pure Appl Chem. 2014;86:545–53.
Craig Duncan QM, Reading M. Thermal analysis of pharmaceuticals. Boca Raton: CRC Press; 2007.
Yoshida MI, Oliveira MA, Gomez ECL, Mussel WN, Castro WV, Soares CDV. Thermal characterization of lovastatin in pharmaceutical formulations. J Therm Anal Calorim. 2011;106:657–64.
Chieng N, Rades T, Aaltonen J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J Pharm Biomed Anal. 2011;55:618–44.
Amorim PHO, Ferreira APG, Machado LCM, Cervini P, Cavalheiro ETG. Investigation on the thermal behavior of beta-blockers antihypertensives atenolol and nadolol using TG/DTG, DTA, DSC, and TG–FTIR. J Therm Anal Calorim. 2015;120:1035–42.
Neto HS, Novák C, Matos J. Thermal analysis and compatibility studies of prednicarbate with excipients used in semi solid pharmaceutical form. J Therm Anal Calorim. 2009;97(1):367–74.
Bannach G, Cervini P, Cavalheiro ETG, Ionashiro M. Using thermal and spectroscopic data to investigate the thermal behavior of epinephrine. Thermochim Acta. 2010;499:123–7.
Bannach G, Arcaro R, Ferroni DC, Siqueira AB, Treu-Filho O, Ionashiro M, Schnitzler E. Thermoanalytical study of some antiinflammatory analgesic agents. J Therm Anal Calorim. 2010;102:163–70.
Ambrozini B, Cervini P, Cavalheiro ETG. Thermal behavior of the beta-blocker propranolol. J Therm Anal Calorim. 2015;. doi:10.1007/s10973-015-5118-7.
Silva ACM, Galico DA, Guerra RB, Legendre AO, Rinaldo D, Galhiane MS, Bannach G. Study of some volatile compounds evolved from the thermal decomposition of atenolol. J Therm Anal Calorim. 2014;115:2517–20.
Correa JCR, Perissinato AG, Serra CHD, Trevisan MG, Salgado HRN. Polymorphic stability of darunavir and its formulation. J Therm Anal Calorim. 2015;. doi:10.1007/s10973-015-4984-3.
Owusu-Ware SK, Cherry AJ, Baltus CB, Spencer J, Antonijevic MD. Thermal analysis of novel biphenylamide derivatives. J Therm Anal Calorim. 2015;121:437–52.
Silva PSP, Castro RAE, Melro E, Silva MR, Maria TMR, Canotilho J, Eusebio MES. Structural evidence of polymorphism and conformational isomorphism of a somewhat flexible molecule: m-anisic acid. J Therm Anal Calorim. 2015;120:667–77.
Neto HS, Novak C, Matos JR. Thermal analysis and compatibility studies of prednicarbate with excipients used in semi solid pharmaceutical form. J Therm Anal Calorim. 2009;97:367–74.
Kumar N, Goindi S, Saini B, Bansal G. Thermal characterization and compatibility studies of itraconazole and excipients for development of solid lipid nanoparticles. J Therm Anal Calorim. 2014;115:2375–83.
Lira AM, Araujo AAS, Basilio IDJ, Santos BLL, Santana DP, Macedo RO. Compatibility studies of lapachol with pharmaceutical excipients for the development of topical formulations. Thermochim Acta. 2007;457:1–6.
de Souza SMM, Franco PIBEM, Leles MIG, da Conceicao EC. Evaluation of thermal stability of enalapril maleate tablets using thermogravimetry and differential scanning calorimetry. J Therm Anal Calorim. 2015;. doi:10.1007/s10973-015-4648-3.
da Silva EP, Pereira MAV, Lima IPD, Lima NGPB, Barbosa EG, Aragao CFS, Gomes APB. Compatibility study between atorvastatin and excipients using DSC and FTIR. J Therm Anal Calorim. 2016;. doi:10.1007/s10973-015-5077-z.
Giron D. Applications of thermal analysis and coupled techniques in pharmaceutical industry. J Therm Anal Calorim. 2002;68:335–57.
Giron D, Goldbronn C. Use of DSC and TG for identification and quantification of the dosage form. J Therm Anal Calorim. 1997;48:473–83.
Etienne S, Becker C, Ruch D, Germain A, Calberg C. Synergetic effect of poly(vinyl butyral) and calcium carbonate on thermal stability of poly(vinyl chloride) nanocomposites investigated by TG–FTIR–MS. J Thermal Anal Calorim. 2010;100:667–77.
Wiecinska P. Thermal degradation of organic additives used in colloidal shaping of ceramics investigated by the coupled DTA/TG/MS analysis. J Thermal Anal Calorim. 2015;. doi:10.1007/s10973-015-5075-1.
Izato Y, Miyake A. Thermal decomposition mechanism of ammonium nitrate and potassium chloride mixtures. J Therm Anal Calorim. 2015;121:287–94.
Zayed MA, Fahmey MA, El-Desawy M, Farrag YS. Structure characterization of terazosin drug using mass spectrometry and thermal analyses techniques in comparison with semi-empirical molecular orbital (MO) calculations. J Therm Anal Calorim. 2015;120:1061–9.
Yılmaz N, Oz S, Atakol A, Svoboda I, Aydiner B, Akay MA, Atakol O. An experimental and theoretical study toward the synthesis, structure and thermal decomposition of some phenyl tetrazoles. J Therm Anal Calorim. 2015;119:2321–8.
Zayed MA, El-Dien FAN, Hawash MF, Fahmey MA. Mass spectra of gliclazide drug at various ion sources temperature. J Therm Anal Calorim. 2010;102:305–12.
Lizarraga E, Zabaleta C, Palop JA. Mechanism of thermal decomposition of thiourea derivatives. J Therm Anal Calorim. 2008;93:887–98.
Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94(3):739–77.
Fernandes AP, Gandin V. Selenium compounds as therapeutic agents in cancer. Biochim Biophys Acta. 2015;1850(8):1642–60.
Nedel F, Campos VF, Alves D, McBride AJ, Dellagostin OA, Collares T, Savegnago L, Seixas FK. Substituted diaryl diselenides: cytotoxic and apoptotic effect in human colon adenocarcinoma cells. Life Sci. 2012;91(9–10):345–52.
Plano D, Baquedano Y, Ibáñez E, Jiménez I, Palop JA, Spallholz JE, Sanmartín C. Antioxidant-prooxidant properties of a new organoselenium compound library. Molecules. 2010;15(10):7292–312.
Romano B, Plano D, Encío I, Palop JA, Sanmartín C. In vitro radical scavenging and cytotoxic activities of novel hybrid selenocarbamates. Bioorg Med Chem. 2015;23(8):1716–27.
Plano D, Baquedano Y, Moreno-Mateos D, Font M, Jiménez-Ruiz A, Palop JA, Sanmartín C. Selenocyanates and diselenides: a new class of potent antileishmanial agents. Eur J Med Chem. 2011;46(8):3315–23.
Baquedano Y, Moreno E, Espuelas S, Nguewa P, Font M, Gutiérrez KJ, Jiménez-Ruiz A, Palop JA, Sanmartín C. Novel hybrid selenosulfonamides as potent antileishmanial agents. Eur J Med Chem. 2014;3(74):116–23.
Chen JN, Wang XF, Li T, Wu DW, Fu XB, Zhang GJ, Shen XC. Wang; HS Design, synthesis, and biological evaluation of novel quinazolinyl-diaryl urea derivatives as potential anticancer agents. Eur J Med Chem. 2016;1(107):12–25.
Wilking MJ, Singh C, Nihal M, Zhong W, Ahmad N. SIRT1 deacetylase is overexpressed in human melanoma and its small molecule inhibition imparts anti-proliferative response via p53 activation. Arch Biochem Biophys. 2014;563:94–100.
Liebman JF, Slayden SW. Thermochemistry of organoselenium and organotellurium compounds. In: Rappoport Z, editor. The chemistry of organic selenium and tellurium compounds, vol. 3. New York: Wiley; 2012. p. 139–65.
Patial BS, Thakur N, Tripathi SK. Crystallization study of Sn additive Se–Te chalcogenide alloys. J Therm Anal Calorim. 2011;106:845–52.
Attanassov PK. Synthesis of 4-(phenylamino)quinazoline-2(1H)-selenoles and diselenides from isoselenocyanates. Helv Chim Acta. 2004;87:1873–87.
Fahmey MA, Zayed MA, El-Shobak HG. Study of some phenolic-iodine redox polymeric products by thermal analyses and mass spectrometry. J Therm Anal Calorim. 2005;82:137–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Díaz, M., Palop, J.A., Sanmartín, C. et al. Thermal stability and decomposition of urea, thiourea and selenourea analogous diselenide derivatives. J Therm Anal Calorim 127, 1663–1674 (2017). https://doi.org/10.1007/s10973-016-5645-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5645-x