Abstract
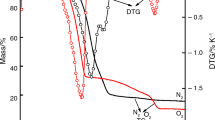
The nonahydrate of iron(III) nitrate shows no phase transitions in the range of −40 to 0 °C. Both hexahydrate Fe(NO3)3·6H2O and nonahydrate Fe(NO3)3·9H2O have practically the same thermal behavior. Thermal decomposition of iron nitrate is a complex process which has a different mechanism than those described for other trivalent elements. Thermolysis begins with the successive condensation of 4 mol of the initial monomer accompanied by the loss of 4 mol of nitric acid. At higher temperature, hydrolytic processes continue with the gradual elimination of nitric acid from resulting tetramer and dimeric iron oxyhydroxide Fe4O4(OH)4 is formed. After complete dehydration, oxyhydroxide is destroyed leaving behind 2 mol of Fe2O3. The molecular mechanics method provides a helpful insight into the structural arrangement of intermediate compounds.












Similar content being viewed by others
References
Gounadali BEL, Kaddami M. H2O+Fe(NO3)3+Ni(NO3)2 ternary system isotherms at 0°C and 30°C. Fluid Phase Equilib. 2011;306:175–80 (and references therein).
Schmidt H, Asztalos A, Bok F, Voigt W. New iron(III) nitrate hydrates: Fe(NO3)3·xH2O with x = 4, 5 and 6. Acta Cryst C. 2012;68:i29–33.
Hair NJ, Beattie JK. Structure of hexaaquairon(III) nitrate trihydrate. Comparison of iron(II) and iron(III) bond lengths in high-spin octahedral environments. Inorg Chem. 1977;16:245–50.
Wieczorek-Ciurowa K, Kozak AJ. The thermal decomposition of Fe(NO3)3·9H2O. J Therm Anal Cal. 1999;58:647–51.
Elmasry MAA, Gaber A, Khater EMH. Thermal decomposition of Ni(II) and Fe(III) nitrates and their mixture. J Therm Anal Cal. 1998;52:489–93.
NIST chemistry WebBook, NIST standard reference database number 69. www.http//webbook.nist/chemistry. Accessed 21 May 2013.
Young DC. Computational chemistry: a practical guide for applying techniques to real-world problems. New York: Wiley; 2001.
http://www.avogadro.openmolecules.net/. Accessed 21 Nov 2012.
Melnikov P, Nascimento VA, Zanoni Consolo LZZ. Thermal decomposition of gallium nitrate hydrate and modeling of thermolysis products. J Therm Anal Cal. 2011;135:1117–21.
Melnikov P, Nascimento VA, Consolo LZZ, Silva AF. Mechanism of thermal decomposition of yttrium nitrate hexahydrate Y(NO3)3·6H2O and modeling of intermediate oxynitrates. J Therm Anal Cal. 2012. doi:10.1007/s10973-012-2236.
Melnikov P, Nascimento VA, Consolo LZZ. Computerized modeling of intermediate compounds formed during thermal decomposition of gadolinium nitrate hydrate. Russ J Phys Chem. 2012;11:1659–63.
Melnikov P, Nascimento VA, Consolo LZZ, de Oliveira LCS. Thermolysis mechanism of chromium nitrate hydrate nonahydrate and computerized modeling of intermediate products. J Therm Anal Cal. doi:10.1007/s10973-013-3106-3.
Boudalis AK, Tangoulis V, Raptopoulou CP, Terzis A, Tuchagues JP, Perlepes SP. A new example of a tetranuclear iron(III) cluster containing the [Fe4O2]8+ core: preparation, X-ray crystal structure, magnetochemistry and Mössbauer study of [Fe4O2(O2CMe)6(N3)2(phen)2]. Inorg Chim Acta. 2004;357:1345–54.
Sutradhar M, Carrlla LM, Rentschler E. A discrete μ4-oxido tetranuclear iron(III) cluster. Eur J Inorg Chem. 2012;2012:4273–78.
Jin MK, Jin Y, Jung D-Y, Heu M, Yoon S, Suh BJ. Crystal packing of two different tetranuclear iron(III) clusters, [(tacn)4Fe4O2(OH)4]2·8Br·9H2O (tacn = 1, 4, 7-triazacyclononane). Bull Korean Chem Soc. 2005;26(2):253–9.
Inorganic crystal structures database, file 262788. Accessed 15 Mar 2013.
Wells AF. Structural inorganic chemistry. 5th ed. London: 327 Clarendon Press, Oxford University Press; 1984.
Acknowledgements
The authors are indebted to CNPq and FUNDECT (Brazilian agencies) for financial support, and to Prof. Dr. H. Schmidt for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melnikov, P., Nascimento, V.A., Arkhangelsky, I.V. et al. Thermal decomposition mechanism of iron(III) nitrate and characterization of intermediate products by the technique of computerized modeling. J Therm Anal Calorim 115, 145–151 (2014). https://doi.org/10.1007/s10973-013-3339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3339-1