Abstract
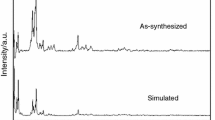
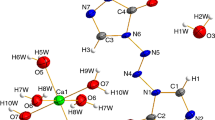
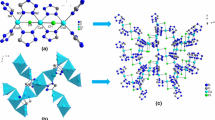
A novel metal organic framework [Co (BTC)1/3 (DMF) (HCOO)] n (CoMOF, BTC = 1,3,5-benzene tricarboxylate, DMF = N,N-dimethylformamide) has been synthesized solvothermally and characterized by single crystal X-ray diffraction, X-ray powder diffraction, and FT-IR spectra. The molar heat capacity of the compound was measured by modulated differential scanning calorimetry (MDSC) over the temperature range from 198 to 418 K for the first time. The thermodynamic parameters such as entropy and enthalpy versus 298.15 K based on the above molar heat capacity were calculated. Moreover, a four-step sequential thermal decomposition mechanism for the CoMOF was investigated through the thermogravimetry and mass spectrometer analysis (TG-DTG-MS) from 300 to 800 K. The apparent activation energy of the first decomposition step of the compound was calculated by the Kissinger method using experimental data of TG analysis.





Similar content being viewed by others
References
Wong-Foy AG, Matzger AJ, Yaghi OM. Exceptional H-2 saturation uptake in microporous metal-organic frameworks. J Am Chem Soc. 2006;128:3494–5.
Fletcher AJ, Thomas KM, Rosseinsky MJ. Flexibility in metal-organic framework materials: impact on sorption properties. J Solid State Chem. 2005;178:2491–510.
Navarro JAR, Barea E, Salas JM, Masciocchi N, Galli S, Sironi A, et al. H2, N2, CO, and CO2 sorption properties of a series of robust sodalite-type microporous coordination polymers. Inorg Chem. 2006;45:2397–9.
Latroche M, Surble S, Serre C, Mellot-Draznieks C, Llewellyn PL, Lee JH, et al. Hydrogen storage in the giant-pore metal-organic frameworks MIL-100 and MIL-101. Angewandte Chem-Int Ed. 2006;45:8227–31.
Wu CD, Hu A, Zhang L, Lin WB. Homochiral porous metal-organic framework for highly enantioselective heterogeneous asymmetric catalysis. J Am Chem Soc. 2005;127:8940–1.
Chen BL, Liang CD, Yang J, Contreras DS, Clancy YL, Lobkovsky EB, et al. A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angewandte Chem-Int Ed. 2006;45:1390–3.
Jalbout AF, Li XH, Hassan MR, Hossain GMG. Construction of novel coordination polymers with simple ligands. Transit Met Chem. 2008;33:597–603.
Reading M, Elliot D, Hill V. Some aspects of the theory and practise of modulated differential scanning calorimetry. In: Proceedings of the 21st North American Thermal Analysis Society Conference. 1992, pp. 145–50.
Wunderlich B. The contributions of MDSC to the understanding of the thermodynamics of polymers. J Therm Anal Calorim. 2006;85:179–87.
Chau J, Garlicka I, Wolf C, Teh J. Modulated DSC as a tool for polyethylene structure characterization. J Therm Anal Calorim. 2007;90:713–9.
Qi YN, Xu F, Ma HJ, Sun LX, Zhang J, Jiang T. Thermal stability and glass transition behavior of PANI/gamma-Al2O3 composites. J Therm Anal Calorim. 2008;91:219–23.
Qiu SJ, Chu HL, Zhang J, Qi YN, Sun LX, Xu F. Heat capacities and thermodynamic properties of CoPc and CoTMPP. J Therm Anal Calorim. 2008;91:841–8.
Zhang J, Zeng JL, Liu YY, Sun LX, Xu F, You WS, et al. Thermal decomposition kinetics of the synthetic complex Pb(1, 4-BDC)center dot(DMF)(H2O). J Therm Anal Calorim. 2008;91:189–93.
Sheldrick GM. SHELX97. Program for crystal structure refinement. 1997.
Archer DG. Thermodynamic properties of synthetic sapphire (Alpha-Al2O3), Standard Reference Material 720 and the effect of temperature-scale differences on thermodynamic properties. J Phys Chem Ref Data. 1993;22:1441–53.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Acknowledgements
The authors gratefully acknowledge the financial support for this study from the National Natural Science Foundation of China (No. 2083309, 20873148, 50671098 and U0734005), the National High Technology Research and Development Program of China (2007AA05Z115 and 2007AA05Z102), the National Basic Research Program (973 program) of China (2010CB631303), and IUPAC (Project No. 2008-006-3-100).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jiang, CH., Song, LF., Zhang, J. et al. Thermodynamic properties and heat capacities of Co (BTC)1/3 (DMF) (HCOO). J Therm Anal Calorim 102, 1087–1093 (2010). https://doi.org/10.1007/s10973-010-0688-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0688-x