Abstract
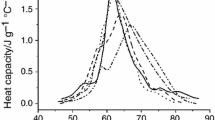
The thermodynamic parameters calculated from measurements obtained by differential scanning calorimetry from healthy and focal segmental glomerulosclerosis albumin are reported. The same values were determined by fluorescence spectra and by the second derivative absorption spectra and they resulted in agreement with values obtained from the calorimetry technique. Nevertheless the unfolding mechanism seems to be completely altered when pathological albumin is compared with healthy albumin. The C p values measured by calorimetry show an increase with mild slope with healthy protein; on the contrary the slope intensely increase with pathological protein. Furthermore the λmax of this molecule is lower and drastically decrease with the increase of temperature when compared with healthy one.
Therefore the modification of cys 34 on pathological albumin is supposed to cause an alteration of the structure, the swelling and the unfolding mechanism.
Similar content being viewed by others
References
GL Braden JG Mulhern MH O’Shea SV Nash AA Ucci Jr. MJ Germain (2000) Am. J. Kidney Dis. 35 878 Occurrence Handle1:STN:280:DC%2BD3c3lsFWitA%3D%3D
SM Korbet (1998) J. Am. Soc. Nephrol. 9 1333 Occurrence Handle1:STN:280:DyaK1czgvVGitQ%3D%3D
M Bruschi L Musante G Candiano L Santucci C Zennaro M Carraro P Del Boccio R Gusmano F Perfumo A Urbani GM Ghiggeri (2006) Electrophoresis 27 2960 Occurrence Handle10.1002/elps.200500641 Occurrence Handle1:CAS:528:DC%2BD28XotVCgtLw%3D
JF Foster et al. (1977) Albumin Structure, Function and Uses Pergamon Press New York
A Kawakami K Kubota N Yamada U Tagami K Takehana I Sonaka E Suzuki K Hirayama (2006) Febs. J. 273 3346 Occurrence Handle10.1111/j.1742-4658.2006.05341.x Occurrence Handle1:CAS:528:DC%2BD28Xot1Gjs7o%3D
G Buhrman B Parker J Sohn J Rudolph C Mattos (2005) Biochemistry 44 5307 Occurrence Handle10.1021/bi047449f Occurrence Handle1:CAS:528:DC%2BD2MXitlSiur0%3D
SJ Leach HA Sherage (1960) J. Am. Chem. Soc. 82 4790 Occurrence Handle10.1021/ja01503a008 Occurrence Handle1:CAS:528:DyaF3MXnvF2jsw%3D%3D
R Ragone G Colonna C Balestrieri L Servillo G Irace (1984) Biochemistry 23 1871 Occurrence Handle10.1021/bi00303a044 Occurrence Handle1:CAS:528:DyaL2cXhtlGhtLo%3D
A Ruiz-Arribas GG Zhadan VP Kutyshenko RI Santamaria M Cortijo E Villar JM Fernandez-Abalos JJ Calvete VL Shnyrov (1998) Eur. J. Biochem. 253 462 Occurrence Handle10.1046/j.1432-1327.1998.2530462.x Occurrence Handle1:CAS:528:DyaK1cXis1Kgsr0%3D
JM Sanchez-Ruiz JL Lopez-Lacomba M Cortijo PL Mateo (1988) Biochemistry 27 1648 Occurrence Handle10.1021/bi00405a039 Occurrence Handle1:CAS:528:DyaL1cXhtVyktbY%3D
JM Sanchez-Ruiz (1992) Biophys. J. 61 921 Occurrence Handle1:CAS:528:DyaK38XitlWhu70%3D Occurrence Handle10.1016/S0006-3495(92)81899-4
E Freire R Biltonen (1978) Biopolymers 17 463 Occurrence Handle10.1002/bip.1978.360170212 Occurrence Handle1:CAS:528:DyaE1cXhtFKksbg%3D
PD Ross A Shrake (1988) J. Biol. Chem. 263 11196 Occurrence Handle1:CAS:528:DyaL1cXltVKktLY%3D
KS Krishinan JF Brandts (1978) Methods Enzymol. 49 3 Occurrence Handle1:STN:280:DyaE1c7ns1eqtA%3D%3D Occurrence Handle10.1016/S0076-6879(78)49003-2
W Pfeil PL Privalov (1976) Biophys. Chem. 4 23 Occurrence Handle10.1016/0301-4622(76)80003-8 Occurrence Handle1:CAS:528:DyaE28XhtFOktrw%3D
JF Brandts (1964) J. Am. Chem. Soc. 86 4291 Occurrence Handle10.1021/ja01074a013 Occurrence Handle1:CAS:528:DyaF2cXkvVGmsbw%3D
JF Brandts (1964) J. Am. Chem. Soc. 86 4302 Occurrence Handle10.1021/ja01074a014 Occurrence Handle1:CAS:528:DyaF2cXkvVGmsb0%3D
YA Gryzunov A Arroyo JL Vigne Q Zhao VA Tyurin CA Hubel RE Gandley YA Vladimirov RN Taylor VE Kagan (2003) Arch. Biochem. Biophys. 413 53 Occurrence Handle10.1016/S0003-9861(03)00091-2 Occurrence Handle1:CAS:528:DC%2BD3sXivV2ns7w%3D
G Pico (1995) Biochem. Mol. Biol. Int. 36 1017 Occurrence Handle1:CAS:528:DyaK28XjtlOitLg%3D
A Shrake PD Ross (1988) J. Biol. Chem. 2 63 15392
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bruschi, M., Musante, L., Ghiggeri, G.M. et al. Comparative study of thermal stability of healthy and focal segmental glomerulosclerosis plasma albumin. J Therm Anal Calorim 87, 27–31 (2007). https://doi.org/10.1007/s10973-006-7822-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-7822-9