Abstract
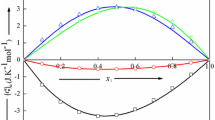
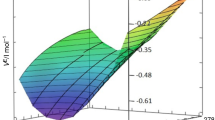
The specific heat capacities of {2-(hexyloxytriethoxy)ethanol (C6E4)+water} system have been measured from 280 to 333 K within the whole composition range by DSC. Changes of specific, apparent and partial molar heat capacities of investigated aqueous solution vs. composition and temperature, considered as an effect of structural transformations were analyzed in order to draw boundary between region where amphiphile molecules occur as monomers and small aggregates and the area in which the first micelles appear. For each solution, the temperature dependences of the differential heat flow were analyzed in order to find the curve of phase coexistence, i.e. the boundary between one- and two-phase areas for the examined system.
Similar content being viewed by others
References
G Roux G Perron JE Desnoyers (1978) J. Solution Chem. 7 639 Occurrence Handle1:CAS:528:DyaE1MXivFyruw%3D%3D Occurrence Handle10.1007/BF00652015
S Smith P Wiseman L Boudreau G Marangoni R Palepu (1994) J. Solution Chem. 23 207 Occurrence Handle1:CAS:528:DyaK2cXivVWjsrY%3D Occurrence Handle10.1007/BF00973547
U Kaatze B Gabriel R Pottel (1994) Ber. Bunsen-ges. Phys. Chem. 98 9 Occurrence Handle1:CAS:528:DyaK2cXitVKhtbk%3D Occurrence Handle10.1002/bbpc.19940980103
G Douhéret A Pal MI Davis (1989) J. Chem. Soc., Faraday Trans. 1, 85 2723 Occurrence Handle10.1039/f19898502723
G Douhéret AH Roux MI Davis ME Hernandez H Høiland E Høgseth (1993) J. Solution Chem. 22 1041 Occurrence Handle10.1007/BF00647728
G Douhéret C Salgado MI Davis J Loya (1992) Thermochim. Acta 207 313 Occurrence Handle10.1016/0040-6031(92)80145-M
L Paduano R Sartorio V Vitagliano L Constantino (1997) J. Colloid Interface Sci. 189 189 Occurrence Handle1:CAS:528:DyaK2sXjslSgtro%3D Occurrence Handle10.1006/jcis.1997.4808
L Ambrosone L Constantino G D’Errico V Vitagliano (1997) J. Colloid Interface Sci. 190 286 Occurrence Handle1:CAS:528:DyaK2sXktlShu70%3D Occurrence Handle10.1006/jcis.1997.4860
SA Wieczorek (2000) J. Chem. Thermodynamics 32 529 Occurrence Handle1:CAS:528:DC%2BD3cXit1CmsL4%3D Occurrence Handle10.1006/jcht.1999.0621
H Piekarski M Tkaczyk M Wasiak (2005) J. Therm. Anal. Cal. 82 711 Occurrence Handle1:CAS:528:DC%2BD28XjsFGjug%3D%3D Occurrence Handle10.1007/s10973-005-0954-5
H Piekarski M Tkaczyk (2005) Thermochim. Acta 428 113 Occurrence Handle1:CAS:528:DC%2BD2MXitlShsbs%3D Occurrence Handle10.1016/j.tca.2004.11.001
T Telgmann U Kaatze (2000) J. Phys. Chem. A 104 4846 Occurrence Handle1:CAS:528:DC%2BD3cXivVWht70%3D Occurrence Handle10.1021/jp994159d
K-V Schubert R Strey M Kahlweit (1991) J. Colloid Interface Sci 141 21 Occurrence Handle1:CAS:528:DyaK3MXosVehtQ%3D%3D Occurrence Handle10.1016/0021-9797(91)90298-M
T Telgmann U Kaatze (2000) J. Phys. Chem. A 104 1085 Occurrence Handle1:CAS:528:DC%2BD3cXlsl2rsw%3D%3D Occurrence Handle10.1021/jp9923116
R Strey (1996) Ber. Bunsen-ges. Phys. Chem. 100 182 Occurrence Handle1:CAS:528:DyaK28Xis1egu7o%3D Occurrence Handle10.1002/bbpc.19961000303
M Corti C Minero V Degiorgio (1984) J. Phys. Chem. 88 309 Occurrence Handle1:CAS:528:DyaL2cXktVSktA%3D%3D Occurrence Handle10.1021/j150646a029
M Kahlweit R Strey P Firman D Haase J Jen R Schomäcker (1988) Langmuir 4 499 Occurrence Handle1:CAS:528:DyaL1cXitFSltrw%3D Occurrence Handle10.1021/la00081a002
MN Garcia-Lisbona A Galindo G Jackson AN Burgess (1998) J. Am. Chem. Soc. 120 4191 Occurrence Handle1:CAS:528:DyaK1cXis12msr0%3D Occurrence Handle10.1021/ja9736525
L Wojtczak H Piekarski M Tkaczyk I Zasada T Rychtelska (2002) J. Mol. Liquids 95 229 Occurrence Handle1:CAS:528:DC%2BD38XhsFantr0%3D Occurrence Handle10.1016/S0167-7322(01)00290-2
P Jablonski A Müller-Blecking W Borchard (2003) J. Therm. Anal. Cal. 74 779. Occurrence Handle10.1023/B:JTAN.0000011010.84094.90
JJ Moura Ramos CAM Afonso LC Branco (2003) J. Therm. Anal. Cal. 71 659 Occurrence Handle1:CAS:528:DC%2BD3sXitVynsb0%3D Occurrence Handle10.1023/A:1022884716750
P Góralski M Tkaczyk M Chorążewski (2003) J. Chem. Eng. Data 48 492 Occurrence Handle10.1021/je020042y
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piekarski, H., Tkaczyk, M. Heat capacity and phase behavior of {C6E4+water} solutions by DSC . J Therm Anal Calorim 83, 541–547 (2006). https://doi.org/10.1007/s10973-005-7496-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-7496-8