Abstract
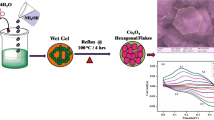
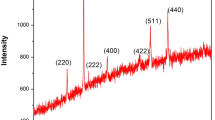
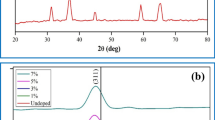
Cobalt Oxide (Co3O4) nanoparticles were synthesized with an aid of urea by sol–gel method. The results of X-ray diffraction revealed that the formation of face-centered cubic (Fd3m) structure and the average crystallite size of the product were found to be 13.76 nm. The formation of cobalt oxide is confirmed by FT-IR analysis. The results of HR-TEM images reveal that Co3O4 nanoparticles were found to have within the range of 13–15 nm. At 3 M KOH, electrochemical analyses were investigated with impedance spectroscopy and an intrinsic pseudo capacitance and the results were reported. The results of Galvanostatic charge-discharge (GCD) tests revealed the capacitive properties of Co3O4 with the highest specific capacitance of 761.25 F g−1.

Highlights
-
The specific capacitance of Co3O4 nanoparticles was found to be 761.25 F g−1 at 11 mA/cm2 current density.
-
The Co3O4 nanoparticles were synthesized by a simple sol–gel method.
-
The crystallite size of Co3O4 nanoparticles was found to be 13.76 nm.






Similar content being viewed by others
References
Guan Q, Cheng J, Wang B et al. (2014) Needle-like Co3O4 anchored on the graphene with enhanced electrochemical performance for aqueous supercapacitors. ACS Appl Mater Interf 6:7626–7632. https://doi.org/10.1021/am5009369
Cheng JP, Zhang J, Liu F (2014) Recent development of metal hydroxides as electrode material of electrochemical capacitors. RSC Adv 4:38893–38917. https://doi.org/10.1039/c4ra06738j
Huang D, Liu H, Li T, Niu Q (2019) Template-free synthesis of NiO skeleton crystal octahedron and effect of surface depression on electrochemical performance. J Sol-Gel Sci Technol 89:511–520. https://doi.org/10.1007/s10971-018-4908-3
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828. https://doi.org/10.1039/c1cs15060j
Xuan D, Chengyang W, Mingming C et al. (2009) Electrochemical performances of nanoparticle Fe3O4/activated carbon supercapacitor using KOH electrolyte solution. J Phys Chem C 113:2643–2646. https://doi.org/10.1021/jp8088269
Kaempgen M, Chan CK, Ma J et al. (2009) Printable thin film supercapacitors using single-walled carbon nanotubes. Nano Lett 9:1872–1876. https://doi.org/10.1021/nl8038579
Feng XY, Shen C, Yu Y et al. (2013) Synthesis and electrochemical properties of sticktight-like and nanosheet Co3O4 particles. J Power Sources 230:59–65. https://doi.org/10.1016/j.jpowsour.2012.12.046
Hu CC, Chang KH, Lin MC, Wu YT (2006) Design and tailoring of the nanotubular arrayed architecture of hydrous RuO2 for next generation supercapacitors. Nano Lett 6:2690–2695. https://doi.org/10.1021/nl061576a
Chen S, Zhu J, Wu X et al. (2010) Graphene oxide MnO2. ACS Nano 4:2822–2830. https://doi.org/10.1021/nn901311t
Vijayakumar S, Nagamuthu S, Muralidharan G (2013) Supercapacitor studies on NiO nanoflakes synthesized through a microwave route. ACS Appl Mater Interf 5:2188–2196. https://doi.org/10.1021/am400012h
Li X, Shao J, Li J et al. (2013) Ordered mesoporous MoO2 as a high-performance anode material for aqueous supercapacitors. J Power Sources 237:80–83. https://doi.org/10.1016/j.jpowsour.2013.03.020
Deori K, Ujjain SK, Sharma RK, Deka S (2013) Morphology controlled synthesis of nanoporous Co3O4 nanostructures and their charge storage characteristics in supercapacitors. ACS Appl Mater Interf 5:10665–10672. https://doi.org/10.1021/am4027482
Shinde VR, Mahadik SB, Gujar TP, Lokhande CD (2006) Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis. Appl Surf Sci 252:7487–7492. https://doi.org/10.1016/j.apsusc.2005.09.004
Yan J, Wei T, Qiao W et al. (2010) Rapid microwave-assisted synthesis of graphene nanosheet/ Co3O4 composite for supercapacitors. Electrochim Acta 55:6973–6978. https://doi.org/10.1016/j.electacta.2010.06.081
Wang G, Shen X, Horvat J et al. (2009) Hydrothermal synthesis and optical, magnetic, and supercapacitance properties of nanoporous cobalt oxide nanorods. J Phys Chem C 113:4357–4361. https://doi.org/10.1021/jp8106149
Tummala R, Guduru RK, Mohanty PS (2012) Nanostructured Co3O4 electrodes for supercapacitor applications from plasma spray technique. J Power Sources 209:44–51. https://doi.org/10.1016/j.jpowsour.2012.02.071
Sun L, Wang L, Tian C et al. (2012) Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv 2:4498–4506. https://doi.org/10.1039/c2ra01367c
Lima-Tenório MK, Ferreira CS, Felix Rebelo QH et al. (2018) Pseudocapacitance properties of Co3O4 nanoparticles synthesized using a modified sol-gel method. Mater Res 21:1–7. https://doi.org/10.1590/1980-5373-MR-2017-0521
Rosario AV, Bulhões LOS, Pereira EC (2006) Investigation of pseudocapacitive properties of RuO2 film electrodes prepared by polymeric precursor method. J Power Sources 158:795–800. https://doi.org/10.1016/j.jpowsour.2005.09.002
Guo J, Chen L, Zhang X et al. (2014) Sol-gel synthesis of mesoporous Co3O4 octahedra toward high-performance anodes for lithium-ion batteries. Electrochim Acta 129:410–415. https://doi.org/10.1016/j.electacta.2014.02.104
Ferreira CS, Passos RR, Pocrifka LA (2014) Synthesis and properties of ternary mixture of nickel/cobalt/tin oxides for supercapacitors. J Power Sources 271:104–107. https://doi.org/10.1016/j.jpowsour.2014.07.164
Packiaraj R, Devendran P, Venkatesh KS et al. (2019) Electrochemical investigations of magnetic Co3O4 nanoparticles as an active electrode for supercapacitor applications. J Supercond Nov Magn 32:2427–2436. https://doi.org/10.1007/s10948-018-4963-6
Binitha NN, Suraja PV, Yaakob Z et al. (2010) Simple synthesis of Co3O4 nanoflakes using a low temperature sol-gel method suitable for photodegradation of dyes. J Sol-Gel Sci Technol 53:466–469. https://doi.org/10.1007/s10971-009-2098-8
Farhadi S, Pourzare K, Sadeghinejad S (2013) Simple preparation of ferromagnetic Co3O4 nanoparticles by thermal dissociation of the [CoII(NH3)6](NO3)2 complex at low temperature. J Nanostructure Chem 3:4–10. https://doi.org/10.1186/2193-8865-3-16
Krishnan G, Kooi BJ, Palasantzas G, et al. (2010) Thermal stability of gas phase magnesium nanoparticles. J Appl Phys https://doi.org/10.1063/1.3305453
Hai Z, Gao L, Zhang Q et al. (2016) Facile synthesis of core-shell structured PANI-Co3O4 nanocomposites with superior electrochemical performance in supercapacitors. Appl Surf Sci 361:57–62. https://doi.org/10.1016/j.apsusc.2015.11.171
Meher SK, Justin P, Rao GR (2011) Microwave-mediated synthesis for improved morphology and pseudocapacitance performance of nickel oxide. ACS Appl. Mater. Interfaces 3: 2063–2073. https://doi.org/10.1021/am200294k
Saravanakumar B, Priyadharshini T, Ravi G et al. (2017) Hydrothermal synthesis of spherical NiCO2O4 nanoparticles as a positive electrode for pseudocapacitor applications. J Sol-Gel Sci Technol 84:297–305. https://doi.org/10.1007/s10971-017-4504-y
Acknowledgements
I would like to thank Sophisticated Test and Instrumentation Centre, Cochin for extending their instrumental technical support of XRD facility and for recording HR-TEM images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Priyadharsini, C.I., Marimuthu, G., Pazhanivel, T. et al. Sol–Gel synthesis of Co3O4 nanoparticles as an electrode material for supercapacitor applications. J Sol-Gel Sci Technol 96, 416–422 (2020). https://doi.org/10.1007/s10971-020-05393-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05393-x