Abstract
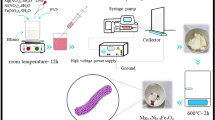
Nickel cobaltite (NCO) is a binary transition-metal oxide, which is extensively used as an electrocatalyst and magnetic material. NCO nanofibers and NCO/graphene composite exhibit high electrochemical reactivity due to the directional bridging of NCO particles. This makes NCO a promising candidate electrode material for use in supercapacitors and batteries. Besides, NCO is also a promising magnetic material due to its unique structural composition, where the cations are seated in octahedral sites surrounded by oxygen vacancies. In the present work, a simple and reliable method was discovered for tuning the morphological and structural changes of nickel cobaltite (NCO) nanoparticles, which were reshaped along the NCO nanofibers, by controlling the pyrolysis soaking time. As the pyrolysis soaking time increases, NCO transforms from inverse spinel to normal spinel; and the morphology of NCO nanoparticles changes from spherical to rod-like. These changes were validated by the hypsochromic peak shifts in Raman, and FTIR spectroscopies. The magnetic measurements reveal changes in the shape of the hysteresis loop, which are explained on the basis of structural and morphological changes in the nanostructure. The net magnetisation increases and coercivity decreases, with an increase in pyrolysis soaking time. These changes in magnetic parameters are attributed to structural changes caused by the formation of oxygen vacancies, and surface effects due to switching in morphology of the NCO nanoparticle.






Similar content being viewed by others
References
Dai Y, Liu W, Formo E, Sun Y, Xia Y (2011) Ceramic nanofibers fabricated by electrospinning and their applications in catalysis, environmental science, and energy technology. Polym Adv Technol 22:326–338. https://doi.org/10.1002/pat.1839
Yuan C, Wu HB, Xie Y, (David) Lou XW (2014) Mixed transition-metal oxides: design, synthesis, and energy-related applications Angew Chem Int Ed 53:1488–1504. https://doi.org/10.1002/anie.201303971
Niu C, Meng J, Wang X, Han C, Yan M, Zhao K, Xu X, Ren W, Zhao Y, Xu L, Zhang Q, Zhao D, Mai L (2015) General synthesis of complex nanotubes by gradient electrospinning and controlled pyrolysis. Nat Commun 6:ncomms8402. https://doi.org/10.1038/ncomms8402
Dubal DP, Gomez-Romero P, Sankapal BR, Holze R (2015) Nickel cobaltite as an emerging material for supercapacitors: An overview. Nano Energy 11:377–399. https://doi.org/10.1016/j.nanoen.2014.11.013
Cui B, Lin H, Li J-B, Li X, Yang J, Tao J (2008) Core–ring structured NiCo2O4 nanoplatelets: synthesis, characterization, and electrocatalytic applications. Adv Funct Mater 18:1440–1447. https://doi.org/10.1002/adfm.200700982
Windisch CF, Exarhos GJ, Ferris KF, Engelhard MH, Stewart DC (2001) Infrared transparent spinel films with p-type conductivity. Thin Solid Films 398:45–52. https://doi.org/10.1016/S0040-6090(01)01302-5
Li Q, Zeng L, Wang J, Tang D, Liu B, Chen G, Wei M (2011) Magnetic mesoporous organic−inorganic NiCo2O4 hybrid nanomaterials for electrochemical immunosensors. ACS Appl Mater Interfaces 3:1366–1373. https://doi.org/10.1021/am200228k
Bitla Y, Chin Y-Y, Lin J-C, Van CN, Liu R, Zhu Y, Liu H-J, Zhan Q, Lin H-J, Chen C-T, Chu Y-H, He Q,(2015) Origin of metallic behavior in NiCo2O4 ferrimagnet Sci Rep 5:15201. https://doi.org/10.1038/srep15201
Marco JF, Gancedo JR, Gracia M, Gautier JL, Ríos EI, Palmer HM, Greaves C, Berry FJ (2001) Cation distribution and magnetic structure of the ferrimagnetic spinel NiCo2O4. J Mater Chem 11:3087–3093. https://doi.org/10.1039/B103135J
Liu M-C,Kong L-B,Lu C,Li X-M,Luo Y-C,Kang L,(2012) A Sol–gel process for fabrication of NiO/NiCo2O4/Co3O4 composite with improved electrochemical behavior for electrochemical capacitors ACS Appl Mater Interfaces 4:4631–4636https://doi.org/10.1021/am301010u
Jadhav HS, Kalubarme RS, Roh J-W, Jung K-N, Shin K-H, Park C-N, Park C-J (2014) Facile and cost effective synthesized mesoporous spinel NiCo2O4 as catalyst for non-aqueous lithium-oxygen batteries. J Electrochem Soc 161:A2188–A2196. https://doi.org/10.1149/2.0771414jes
Gao X, Zhang H, Li Q, Yu X, Hong Z, Zhang X, Liang C, Lin Z (2016) Hierarchical NiCo2O4 hollow microcuboids as bifunctional electrocatalysts for overall water-splitting. Angew Chem Int Ed 55:6290–6294. https://doi.org/10.1002/anie.201600525
Verma S, Joshi HM, Jagadale T, Chawla A, Chandra R, Ogale S (2008) Nearly monodispersed multifunctional NiCo2O4 spinel nanoparticles: magnetism, infrared transparency, and radiofrequency absorption. J Phys Chem C 112:15106–15112. https://doi.org/10.1021/jp804923t
Garg N, Basu M, Upadhyaya K, Shivaprasad SM, Ganguli AK (2013) Controlling the aspect ratio and electrocatalytic properties of nickel cobaltite nanorods. RSC Adv 3:24328–24336. https://doi.org/10.1039/C3RA44156C
Verma S, Kumar A, Pravarthana D, Deshpande A, Ogale SB, Yusuf SM (2014) Off-stoichiometric nickel cobaltite nanoparticles: Thermal stability, magnetization, and neutron diffraction studies. J Phys Chem C 118:16246–16254. https://doi.org/10.1021/jp504538y
Babu GA, Ravi G, Hayakawa Y (2015) Microwave synthesis and effect of CTAB on ferromagnetic properties of NiO, Co3O4 and NiCo2O4 nanostructures. Appl Phys A 119:219–232. https://doi.org/10.1007/s00339-014-8951-9
Umeshbabu E, Rajeshkhanna G, Justin P, Rao GR (2015) Magnetic, optical and electrocatalytic properties of urchin and sheaf-like NiCo2O4 nanostructures. Mater Chem Phys 165:235–244. https://doi.org/10.1016/j.matchemphys.2015.09.023
Yang X, Yu X, Yang Q, Zhao D, Zhang K, Yao J, Li G, Zhou H, Zuo X (2017) Controllable synthesis and magnetic properties of hydrothermally synthesized NiCo2O4 nano-spheres. Ceram Int 43:8585–8589. https://doi.org/10.1016/j.ceramint.2017.03.121
Nakate UT, Kale SN (2016) Microwave assisted synthesis and characterizations of NiCo2O4 nanoplates and electrical, magnetic properties, Mater. Today Proc 3:1992–1998. https://doi.org/10.1016/j.matpr.2016.04.101
Kumar BS, Gudla VC, Ambat R, Kalpathy SK, Anandhan S (2018) A Mechanistic study on the structure formation of NiCo2O4 nanofibers decorated with in-situ formed graphene-like structures. J Inorg Organomet Polym Mater. (Submitted). https://doi.org/10.1007/s10904-018-0842-7
Kumar BS, Kalpathy SK, Anandhan S (2018) Synergism of fictitious forces on nickel cobaltite nanofibers: electrospinning forces revisited. Phys Chem Chem Phys 20:5295–5304. https://doi.org/10.1039/C7CP07435B
Li L, Peng S, Cheah Y, Teh P, Wang J, Wee G, Ko Y, Wong C, Srinivasan M (2013) Electrospun porous NiCo2O4 Nanotubes as advanced electrodes for electrochemical capacitors. Chem–Eur J 19:5892–5898. https://doi.org/10.1002/chem.201204153
Secula EM (2010) Resistivity and hall measurements, NIST. https://www.nist.gov/pml/engineering-physics-division/resistivity-and-hall-measurements (accessed 19 July 2017).
Chien H-C, Cheng W-Y, Wang Y-H, Wei T-Y, Lu S-Y (2011) Ultralow overpotentials for oxygen evolution reactions achieved by nickel cobaltite aerogels. J Mater Chem 21:18180–18182. https://doi.org/10.1039/C1JM14025F
Venkatachalam V, Alsalme A, Alghamdi A, Jayavel R (2017) Hexagonal-like NiCo2O4 nanostructure based high-performance supercapacitor electrodes. Ionics 23:977–984. https://doi.org/10.1007/s11581-016-1868-x
Arora AK, Rajalakshmi M, Thoguluva R, Sivasubramanian V (2007) Raman spectroscopy of optical phonon confinement in nanostructured materials. J Raman Spectrosc 38:604–617. https://doi.org/10.1002/jrs.1684
Bahlawane N, Ngamou PHT, Vannier V, Kottke T, Heberle J, Kohse-Höinghaus K (2009) Tailoring the properties and the reactivity of the spinel cobalt oxide. Phys Chem Chem Phys 11:9224–9232. https://doi.org/10.1039/B910707J
Ryu SR, Noda I, Jung YM (2010) What is the Origin of Positional Fluctuation of Spectral Features: True Frequency Shift or Relative Intensity Changes of Two Overlapped Bands? Appl Spectrosc 64:1017–1021. 0003-7028/10/6409-1017
Allen GC, Paul M (1995) Chemical characterization of transition metal spinel-type oxides by infrared spectroscopy. Appl Spectrosc 49:451–458. https://doi.org/10.1366/0003702953964372
Windisch CF, Exarhos GJ, Owings RR (2004) Vibrational spectroscopic study of the site occupancy distribution of cations in nickel cobalt oxides. J Appl Phys 95:5435–5442. https://doi.org/10.1063/1.1699505
Preudhomme J, Tarte P (1972) Infrared studies of spinels—IV: Normal spinels with a high-valency tetrahedral cation. Spectrochim Acta Part Mol Spectrosc 28:69–79. https://doi.org/10.1016/0584-8539(72)80013-8
Srivastava AK, Mongia N (2016) Superparamagentic behaviour of MgFe2O4 nano-ferrite. Sci Eng Appl 1:1–5. https://doi.org/10.26705/SAEA.2016.1.1.1-5
McCloy JS, Jiang W, Bennett W, Engelhard M, Lindemuth J, Parmar N, Exarhos GJ (2015) Electrical and magnetic properties modification in heavy ion irradiated nanograin NixCo(3–x)O4 films. J Phys Chem C 119:22465–22476. https://doi.org/10.1021/acs.jpcc.5b06406
Tang J, Alivisatos AP (2006) Crystal splitting in the growth of Bi2S3. Nano Lett 6:2701–2706. https://doi.org/10.1021/nl0615930
Sontu UB, Yelasani V, Musugu VRR (2015) Structural, electrical and magnetic characteristics of nickel substituted cobalt ferrite nano particles, synthesized by self combustion method. J Magn Magn Mater 374:376–380. https://doi.org/10.1016/j.jmmm.2014.08.072
McHenry ME, Majetich SA, Kirkpatrick EM (1995) Synthesis, structure, properties and magnetic applications of carbon-coated nanocrystals produced by a carbon arc. Mater Sci Eng A 204:19–24. https://doi.org/10.1016/0921-5093(95)09930-1
Silambarasan M, Ramesh PS, Geetha D (2017) Facile one-step synthesis, structural, optical and electrochemical properties of NiCo2O4 nanostructures. J Mater Sci Mater Electron 28:323–336. https://doi.org/10.1007/s10854-016-5527-9
Liu Z-Q, Xiao K, Xu Q-Z, Li N, Su Y-Z, Wang H-J, Chen S (2013) Fabrication of hierarchical flower-like super-structures consisting of porous NiCo2O4 nanosheets and their electrochemical and magnetic properties. RSC Adv 3:4372–4380. https://doi.org/10.1039/C3RA23084H
Coey JMD, Venkatesan M, Fitzgerald CB (2005) Donor impurity band exchange in dilute ferromagnetic oxides. Nat Mater 4:173–179. https://doi.org/10.1038/nmat1310
Su Y-Z, Xu Q-Z, Chen G-F, Cheng H, Li N, Liu Z-Q (2015) One dimensionally spinel NiCo2O4 nanowire arrays: facile synthesis, water oxidation, and magnetic properties. Electrochim Acta 174:1216–1224. https://doi.org/10.1016/j.electacta.2015.06.092
Zhao J, Cheng Y, Yan X, Sun D, Zhu F, Xue Q (2012) Magnetic and electrochemical properties of CuFe2O4 hollow fibers fabricated by simple electrospinning and direct annealing. CrystEngComm 14:5879–5885. https://doi.org/10.1039/C2CE25684C
Chen X, Unruh KM, Ni C, Ali B, Sun Z, Lu Q, Deitzel J, Xiao JQ (2011) Fabrication, formation mechanism, and magnetic properties of metal oxide nanotubes via electrospinning and thermal treatment. J Phys Chem C 115:373–378. https://doi.org/10.1021/jp1082533
Jiang JZ, Goya GF, Rechenberg HR (1999) Magnetic properties of nanostructured CuFe2O4. J Phys Condens Matter 11:4063. https://doi.org/10.1088/0953-8984/11/20/313
Razaul KM, Hideaki S, Mina N, Kazuto H, Hidenobu K, Takeshi M, Takaaki T, Michio K, Keita K, Mohamedally K, Yasumichi M, Shinya H (2012) Electrical conductivity and ferromagnetism in a reduced graphene–metal oxide hybrid. Adv Funct Mater 23:323–332. https://doi.org/10.1002/adfm.201201418
Iliev MN, Silwal P, Loukya B, Datta R, Kim DH, Todorov ND, Pachauri N, Gupta A (2013) Raman studies of cation distribution and thermal stability of epitaxial spinel NiCo2O4 films. J Appl Phys 114:033514. https://doi.org/10.1063/1.4815874
Ndione PF, Shi Y, Stevanovic V, Lany S, Zakutayev A, Parilla PA, Perkins JD, Berry JJ, Ginley DS, Toney MF (2013) Control of the electrical properties in spinel oxides by manipulating the cation disorder. Adv Funct Mater 24:610–618. https://doi.org/10.1002/adfm.201302535.
Shi X, Bernasek SL, Selloni A (2016) Formation, electronic structure, and defects of Ni substituted spinel cobalt oxide: a DFT + U study. J Phys Chem C 120:14892–14898. https://doi.org/10.1021/acs.jpcc.6b03096
Silwal P, La-o-vorakiat C, Chia EEM, Kim DH, Talbayev D (2013) Effect of growth temperature on the terahertz-frequency conductivity of the epitaxial transparent conducting spinel NiCo2O4 films. AIP Adv 3:092116. https://doi.org/10.1063/1.4821548
Tareen JAK, Małecki A, Doumerc JP, Launay JC, Dordor P, Pouchard M, Hagenmuller P (1984) Growth and electrical properties of pure and Ni-doped Co3O4 single crystals. Mater Res Bull 19:989–997. https://doi.org/10.1016/0025-5408(84)90212-5
Appandairajan NK, Gopalakrishnan J (1978) A study of Co3−xNixO4 (O ≤ x ≤ 1) system. Proc Indian Acad Sci-Sect Chem Sci 87:115–120. https://doi.org/10.1007/BF03182122
Makhlouf SA, Bakr ZH, Al-Attar H, Moustafa MS (2013) Structural, morphological and electrical properties of Cr2O3 nanoparticles. Mater Sci Eng B 178:337–343. https://doi.org/10.1016/j.mseb.2013.01.012
George G, Anandhan S (2014) Synthesis and characterisation of nickel oxide nanofibre webs with alcohol sensing characteristics. RSC Adv 4:62009–62020. https://doi.org/10.1039/C4RA11083H
Wu Z-S, Zhou G, Yin L-C, Ren W, Li F, Cheng H-M (2012) Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 1:107–131. https://doi.org/10.1016/j.nanoen.2011.11.001
Wu M, Meng S, Wang Q, Si W, Huang W, Dong X (2015) Nickel–cobalt oxide decorated three-dimensional graphene as an enzyme mimic for glucose and calcium detection. ACS Appl Mater Interfaces 7:21089–21094. https://doi.org/10.1021/acsami.5b06299
Windisch CF, Ferris KF, Exarhos GJ (2001) Synthesis and characterization of transparent conducting oxide cobalt–nickel spinel films. J Vac Sci Technol Vac Surf Films 19:1647–1651. https://doi.org/10.1116/1.1351799
Mohan VB, Brown R, Jayaraman K, Bhattacharyya D (2015) Characterisation of reduced graphene oxide: Effects of reduction variables on electrical conductivity. Mater Sci Eng B 193:49–60. https://doi.org/10.1016/j.mseb.2014.11.002
Acknowledgements
SK Balakrishnamurthy is obliged to National Institute of Technology Karnataka, India, for a research fellowship. The testing and characterisation was partially supported by the new faculty initiation grant (No: MET/15-16/836/NFIG/SRER), sanctioned to SK Kalpathy from the Industrial Consultancy and Sponsored Research, Indian Institute of Technology Madras, Chennai, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
-
Nickel cobaltite (NCO) nanofibers with in situ formed graphene-like structure.
-
Evidence of inverse to normal spinel transformation in NCO crystal structure.
-
Change in coercivity of NCO nanofibers as a function of NCO nanoparticle shape.
-
Synergism of NCO nanoparticle and graphene-like structure on electrical resistivity.
Rights and permissions
About this article
Cite this article
Kumar, B.S., Dhanasekhar, C., Venimadhav, A. et al. Pyrolysis-controlled synthesis and magnetic properties of sol–gel electrospun nickel cobaltite nanostructures. J Sol-Gel Sci Technol 86, 664–674 (2018). https://doi.org/10.1007/s10971-018-4672-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4672-4