Abstract
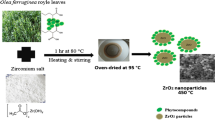
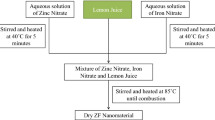
Zirconia nanoparticles with the cubic phase were prepared from zirconium acetate and lemon juice as the precursors. Then, the effect of sucrose addition on improving the particle size and the agglomeration of product was investigated. The particle size of as-synthesized nanoparticles was obtained using FESEM. The results showed that the as-obtained product with the mix of lemon juice (20 mL) and sucrose had a better morphology with the mean particle size of about 21 nm. These nanoparticles were selected and further characterized by EDS, AAS, XRD, UV–Vis, and PL spectroscopy. The EDS and AAS revealed the presence of Mg and Ca in the sample introduced from lemon juice. Also, XRD confirmed the formation of the cubic-phase zirconia. The synthesized cubic ZrO2 nanoparticles exhibited a broad photoluminescence in the UV–Vis region. Then, a pellet from these nanoparticles was prepared and the electrical property of this sample was measured in the temperature range of 450–750 °C using four-probe techniques. The results revealed that these zirconia nanoparticles could have a potential application as an electrolyte material in the intermediate-temperature solid oxide fuel cells.
Graphical Abstract













Similar content being viewed by others
References
Zink N et al (2013) Low temperature synthesis of monodisperse nanoscaled ZrO2 with a large specific surface area. Dalton Trans 42:432
Davar F, Hassankhani A, Loghman-Estarki MR (2013) Controllable synthesis of metastable tetragonal zirconia nanocrystals using citric acid assisted sol–gel method. Ceram Int 39:2933–2941
Loghman-Estarki MR et al (2013) Comparative studies on synthesis of nanocrystalline Sc2O3–Y2O3 doped zirconia (SYDZ) and YSZ solid solution via modified and classic Pechini method. CrystEngComm 15:5898
Loghman-Estarki MR, Nejati M, Edris H, Razavi RS, Jamai H (2015) Evaluation of hot corrosion behavior of plasma sprayed scandia and yttria co-stabilized nanostructured thermal barrier coatings in the presence of molten sulfate and vanadate salt. J Eur Ceram Soc 35:693–702
Tsunekawa S et al (2003) Critical size of the phase transition from cubic to tetragonal in pure zirconia nanoparticles. Nano Lett 3:871
Serena S, Caballero A, Sainz MA (2013) Analysis of the polymorphic transformation of nano-and microcrystalline zirconia doped with CaO and MgO during reaction-sintering process by neutron thermodiffraction. A thermodynamic approach. J Eur Ceram Soc 33:1413
Nath S, Sinha N, Basu B (2008) Microstructure, mechanical and tribological properties of microwave sintered calcia-doped zirconia for biomedical applications. Ceram Int 34:1509
Flegler AJ et al (2014) Cubic yttria stabilized zirconia sintering additive impacts: a comparative study. Ceram Int 40:16323
Petrik NG, Taylor DP, Orlando TM (1999) Laser-stimulated luminescence of yttria-stabilized cubic zirconia crystals. J Appl Phys 85:6770
Tan D et al (2009) Preparation of zirconia nanoparticles by pulsed laser ablation in liquid. Chem Lett 38:1102
Brossmann U et al (2007) Microwave plasma synthesis of nano-crystalline YSZ. Phys Status Solidi (RRL) Rapid Res Lett 1:107
Salavati-Niasari M, Dadkhah M, Davar F (2009) Pure cubic ZrO2 nanoparticles by thermolysis of a new precursor. Polyhedron 28:3005
Meetei SD, Singh SD (2014) Hydrothermal synthesis and white light emission of cubic ZrO2:Eu3+ nanocrystals. J Alloys Compd 587:143
Majedi A, Davar F, Abbasi A (2014) Sucrose-mediated sol–gel synthesis of nanosized pure and S-doped zirconia and its catalytic activity for the synthesis of acetyl salicylic acid. J Ind Eng Chem 20:4215
Barrera-Solano C et al (1994) Obtaining and sintering yttria stabilized zirconia (YSZ) powders from alkoxides. J Sol-Gel Sci Technol 2:347
Răileanu M et al (2015) Sol-gel zirconia-based nanopowders with potential applications for sensors. Ceram Int 41:4381
Liu Y et al (2012) Amine-functionalized lanthanide-doped zirconia nanoparticles: optical spectroscopy, time-resolved fluorescence resonance energy transfer biodetection, and targeted imaging. J Am Chem Soc 134:15083
Hench LL, West JK (1990) The sol-gel process. Chem Rev 90:33
Lin C, Zhang C, Lin J (2007) Phase transformation and photoluminescence properties of nanocrystalline ZrO2 powders prepared via the Pechini-type sol–gel process. J Phys Chem C 111:3300
Razpotnik T, Maček J (2007) Synthesis of nickel oxide/zirconia powders via a modified Pechini method. J Eur Ceram Soc 27:1405
Laberty-Robert C et al (2002) Synthesis of YSZ powders by the sol-gel method: surfactant effects on the morphology. Solid State Sci 4:1053
Kumar PPNV et al (2014) Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity. Ind Crops Prod 52:562
Bala N et al (2015) Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv 5:4993
Lorente J et al (2014) Chemical guide parameters for Spanish lemon (Citrus limon (L.) Burm.) juices. Food Chem 162:186
Dimri MC et al (2012) Room-temperature ferromagnetism in Ca and Mg stabilized cubic zirconia bulk samples and thin films prepared by pulsed laser deposition. J Phys D Appl Phys 45:475003
Singh KA, Pathak LC, Roy SK (2007) Effect of citric acid on the synthesis of nano-crystalline yttria stabilized zirconia powders by nitrate–citrate process. Ceram Int 33:1463
Guo G-Y, Chen Y-L (2001) High-quality zirconia powder resulting from the attempted separation of acetic acid from acrylic acid with zirconium oxychloride. J Mater Chem 11:1283
Sarkar D et al (2007) Synthesis and characterization of sol–gel derived ZrO2 doped Al2O3 nanopowder. Ceram Int 33:1275
Dawber JG, Brown DR, Reed RA (1966) Acid-catalyzed hydrolysis of sucrose: a student study of a reaction mechanism. J Chem Educ 43:34
Joseph AM (1989) Thermal decomposition of carbohydrates, in thermal generation of aromas. American Chemical Society, Washington, p 32
Sahu HR, Rao GR (2000) Characterization of combustion synthesized zirconia powder by UV–vis, IR and other techniques. Bull Mater Sci 23:349
Emeline A et al (1998) Spectroscopic and photoluminescence studies of a wide band gap insulating material: powdered and Colloidal ZrO2 sols. Langmuir 14:5011
Xu X, Wang X (2009) Fine tuning of the sizes and phases of ZrO2 nanocrystals. Nano Res 2:891
Reddy KM, Manorama SV, Reddy AR (2003) Bandgap studies on anatase titanium dioxide nanoparticles. Mater Chem Phys 78:239
Choi S-I, Takeuchi T (1983) Electronic states of F-type centers in oxide crystals: a new picture. Phys Rev Lett 50:1474
Liang J et al (2002) Photoluminescence of tetragonal ZrO2 nanoparticles synthesized by microwave irradiation. Inorg Chem 41:3602
Lee DS et al (2005) Characterization of ZrO2 co-doped with Sc2O3 and CeO2 electrolyte for the application of intermediate temperature SOFCs. Solid State Ion 176:33
Yao M et al (2014) The influence of titanium doping on the electric properties of amorphous alumina films prepared by sol–gel technology. J Sol-Gel Sci Technol 74:39
Kulkarni S, Duttagupta S, Phatak G (2014) Sol–gel synthesis and protonic conductivity of yttrium doped barium cerate. J Sol-Gel Sci Technol 74:94
Kilner JA, Burriel M (2014) Materials for intermediate-temperature solid-oxide fuel cells. Annu Rev Mater Res 44:365
Shao Z, Zhou W, Zhu Z (2012) Advanced synthesis of materials for intermediate-temperature solid oxide fuel cells. Prog Mater Sci 57:804
Rayment C, Sherwin S (2003) Introduction to fuel cell technology. Department of Aerospace and Mechanical Engineering, University of Notre Dame, Notre Dame, p 1
Milewski J, Miller A (2006) Influences of the type and thickness of electrolyte on solid oxide fuel cell hybrid system performance. J Fuel Cell Sci Technol 3:396
Kumar B et al (2005) Electrical properties of heterogeneously doped yttria stabilized zirconia. J Power Sources 140:12
Guo X, Ding Y (2004) Grain boundary space charge effect in zirconia: experimental evidence. J Electrochem Soc 151:J1
Mondal P et al (1999) Enhanced specific grain boundary conductivity in nanocrystalline Y2O3-stabilized zirconia. Solid State Ion 118:331
Hui S et al (2007) A brief review of the ionic conductivity enhancement for selected oxide electrolytes. J Power Sources 172:493
Acknowledgments
We are grateful to the University of Tehran for the financial support. We also appreciate the contribution from the Research Council of Isfahan University of Technology (IUT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Majedi, A., Abbasi, A. & Davar, F. Green synthesis of zirconia nanoparticles using the modified Pechini method and characterization of its optical and electrical properties. J Sol-Gel Sci Technol 77, 542–552 (2016). https://doi.org/10.1007/s10971-015-3881-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3881-3