Abstract
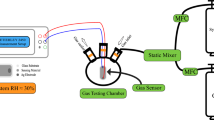
A modified sol–gel method was used to prepare cobalt doped silica thin film with a cobalt content of 10, 20 and 30 mol% (10Co, 20Co and 30Co). The prepared films were annealed at different temperatures in the range 400–1,000 °C, and their structural evolution examined. The mixed valence cobalt oxide, Co3O4, crystallizes only in the sample with the higher cobalt content, while cobalt silicate is the only crystalline phase detected in the sample 10Co and 20Co. Both the cobalt content and the temperature of heat treatment resulted to affect the nature of cobalt species dispersed in the silica matrix. The 30Co was selected for further investigations by FTIR spectroscopy to follow the structural evolution of 30Co film as function of the temperature and UV–Vis to get information on the cobalt valence state. The optical gas-sensing properties of 30Co films, containing Co3O4 as the major cobalt phase, were studied through the measuring of the film transmittance in dry air and in presence of dry air containing variable concentrations of polluting gases, CO and NO2. The 30Co samples resulted to be highly sensitive to CO at room temperature. An explanation for the CO sensing characteristics, at low temperature, was proposed by referring to the physisorption-related mechanics of CO.





Similar content being viewed by others
References
Svegl F, Orel B, Hutchins M (1997) J Sol–Gel Sci Tech 8:765
Martyanov IN, Uma S, Rodrigues S, Klabunde KJ (2005) Langmuir 21:2273
Dutta P, Elbashir NO, Manivannan A, Seehra MS, Roberts CB (2004) Catal Lett 98:203
Cattaruzza E, Battaglin G, Canton P, de Julian Fernandez C, Ferroni M, Gonnella F, Maurizio C, Riello P, Sada C, Sangregorio C, Scremin BF (2004) Appl Surf Sci 226:62
Mergler YJ, van Aalst A, van Delft J, Nieuwenhuys BE (1996) J Catal 161:310
Hamada H, Kintaichi Y, Inaba M, Tanaba M, Yoshinari T, Tsuchida H (1996) Catal Today 29:53
Jansson J (2000) J Catal 194:55
Torncrona A, Skoglundh M, Thormahlen P, Fridell E, Jobson E (1997) Appl Catal B 14:131
Choy JH, Jung H, Han YS, Yoon JB, Shul YG, Kim HJ (2002) Chem Mater 14:3823
Ando M, Kobayashi T, Haruta M (1996) Sensors and Actuators B 32:157
Ando M, Kobayashi T, Haruta M (1997) Catal Today 36:135
Koshizaki N, Yasumoto K, Sasaki T (2000) Sensors and Actuators B 66:122
Cantalini C, Post M, Buso D, Guglielmi M, Martucci A (2005) Sensors and Actuators B 108:184
Martucci A, Buso D, Guglielmi M, Zbroniec L, Koshizaki N, Post M (2004) J Sol–Gel Sci Tech 32:243
Ando M (2006) Trends Anal Chem 23:937
Hwang W-J, Shin K-S, Roh J-H, Lee D-S, Choa S-H (2011) Sensors 11:2580
Jime′nez-Cadena G, Riu J, Xavier Rius F (2007) Analyst 132:1083
Brinker CJ, Scherer GW (1990) Sol–Gel Science: the physics and chemistry of sol–gel processing. Academic Press, New York
Jer′onimo PCA, Ara′ujo AN, Conceicao M, Montenegro BSM (2007) Talanta 72:13
Esposito S, Turco M, Ramis G, Bagnasco G, Pernice P, Pagliuca C, Bevilacqua M, Aronne A (2007) J Solid State Chem 180:3341
Clayden NJ, Pernice P, Aronne A (2005) J Non-Cryst Solids 351:195
Lavrenčič Štangar U, Orel B, Krajnc M (2003) J Sol-Gel Sci Tech 26:771
Lenglet M, Jorgensen CK (1994) Chem Phys Lett 229:616
Armelao L, Barreca D, Gross S, Martucci A, Tieto M, Tondello E (2001) J Non-Cryst Solids 293–295:477
Ortega-Zarzosa G, Araujo-Andrade C, Compeàn-Jasso ME, Martìnez JR (2002) J Sol-Gel Sci Tech 24:23
Rao KJ, Benqlilou-Moudden H, Desbat B, Vinatier P, Levasseur A (2002) J Solid State Chem 165:42
Almeida RM, Pantano CG (1990) J Appl Phys 68:4225
Jansson J, Skoglundh M, Fridell E, Thormählen P (2001) Top Catal 16/17:385
Henrich VE, Cox PA (1996) The surface science of metal oxides. Cambridge University Press, Cambridge
Zeiss H (1960) Organometallic chemistry, Reinhold Pub Corp
Wiberg N, Holleman AF, Wiberg E (2001) Inorganic chemistry. Academic Press, New York
Acknowledgments
Special thanks to Dr. Katayoun Gharagozloo-Hubmann for her useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esposito, S., Setaro, A., Maddalena, P. et al. Synthesis of cobalt doped silica thin film for low temperature optical gas sensor. J Sol-Gel Sci Technol 60, 388–394 (2011). https://doi.org/10.1007/s10971-011-2483-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2483-y