Abstract
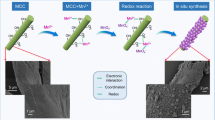
Eco-friendly and low-cost composite, amidoxime-functionalized microcrystalline cellulose/mesoporous silica (MCC/MS-AO), were synthesized by co-condensation method. The obtained materials were characterized by scanning electron microscope (SEM), energy dispersive spectrometer (EDS), Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and N2 adsorption–desorption analysis. Batch experiments were carried out to study the adsorption capacity of U(VI) on MCC/MS-AO. The results show that the material has good adsorption capabilities for U(VI) including high capacity, good selectivity, and reusability. The adsorption process conforms to pseudo-second-order kinetic model and Langmuir model, and it is spontaneous and endothermic. Finally, the possible adsorption mechanism was further studied by X-ray photoelectron spectroscopy (XPS) techniques.
Graphical abstract


















Similar content being viewed by others
Abbreviations
- C 0 :
-
Initial concentration of U(VI) in the solution, mg L−1
- C e :
-
Concentration of U(VI) in the solution after adsorption, mg L−1
- ΔG 0 :
-
Standard Gibbs free energy, kJ mol−1
- ΔH 0 :
-
Standard enthalpy, kJ mol−1
- K 1 :
-
Adsorption rate constants for pseudo-first-order kinetic model, min−1
- K 2 :
-
Adsorption rate constants for pseudo-second-order kinetic model, min−1
- K d :
-
Distribution constant, mL g−1
- K F :
-
Freundlich constant, which is related to adsorption capacity and adsorption strength, mg1−n Ln g−1
- K L :
-
Langmuir constant, which is related to the affinity of the binding site and the free energy of adsorption, L mg−1
- m:
-
Quantity of adsorbent, mg
- n :
-
Freundlich linearity index
- q e :
-
Adsorption capacity at equilibrium state, mg g−1
- q m :
-
Maximum adsorption capacity, mg g−1
- q t :
-
Adsorption capacity at any time, mg g−1
- R :
-
Thermodynamic constant, 8.314 J mol−1 K−1
- R L :
-
Separation factor
- ΔS 0 :
-
Standard entropy, J mol−1 K−1
- S U/M :
-
Selectivity coefficient
- t :
-
Adsorption time, min
- T :
-
Temperature, K
- V :
-
Volume of U(VI) containing solution, mL
- η :
-
Adsorption efficiency
References
Rahman ROA, Ibrahium HA, Hung YT (2011) Liquid radioactive wastes treatment: a review. Water 3(2):551–565. https://doi.org/10.3390/w3020551
Amphlett JTM, Ogden MD, Foster RI, Syna N, Soldenhoff K, Sharrad CA (2018) Polyamine functionalised ion exchange resins: synthesis, characterisation and uranyl uptake. Chem Eng J 334:1361–1370. https://doi.org/10.1016/j.cej.2017.11.040
Raff O, Wilken R-D (1999) Removal of dissolved uranium by nanofiltration. Desalination 122(2):147–150. https://doi.org/10.1016/S0011-9164(99)00035-1
Senol A (2014) Optimization of extractive removal of uranium(VI) from aqueous acidic solutions using commercial amines: Linear solvation energy relation based modeling. Sep Purif Technol 131:35–49. https://doi.org/10.1016/j.seppur.2014.04.034
Cui M, Jang M, Kang K, Kim D, Snyder SA, Khim J (2016) A novel sequential process for remediating rare-earth wastewater. Chemosphere 144:2081–2090. https://doi.org/10.1016/j.chemosphere.2015.10.107
He H, Zong M, Dong F, Yang P, Ke G, Liu M, Nie X, Ren W, Bian L (2017) Simultaneous removal and recovery of uranium from aqueous solution using TiO2 photoelectrochemical reduction method. J Radioanal Nucl Chem 313(1):59–67. https://doi.org/10.1007/s10967-017-5278-y
Anirudhan TS, Lekshmi GS, Shainy F (2019) Synthesis and characterization of amidoxime modified chitosan/bentonite composite for the adsorptive removal and recovery of uranium from seawater. J Colloid Interface Sci 534:248–261. https://doi.org/10.1016/j.jcis.2018.09.009
Xue G, Yurun F, Li M, Dezhi G, Jie J, Jincheng Y, Haibin S, Hongyu G, Yujun Z (2017) Phosphoryl functionalized mesoporous silica for uranium adsorption. Appl Surf Sci 402:53–60. https://doi.org/10.1016/j.apsusc.2017.01.050
Huang G, Peng W, Yang S (2018) Synthesis of magnetic chitosan/graphene oxide nanocomposites and its application for U(VI) adsorption from aqueous solution. J Radioanal Nucl Chem 317(1):337–344. https://doi.org/10.1007/s10967-018-5850-0
Liu X, Li J, Wang X, Chen C, Wang X (2015) High performance of phosphate-functionalized graphene oxide for the selective adsorption of U(VI) from acidic solution. J Nucl Mater 466:56–64. https://doi.org/10.1016/j.jnucmat.2015.07.027
Luo W, Xiao G, Tian F, Richardson JJ, Wang Y, Zhou J, Guo J, Liao X, Shi B (2019) Engineering robust metal–phenolic network membranes for uranium extraction from seawater. Energy Environ Sci 12(2):607–614. https://doi.org/10.1039/c8ee01438h
Mahfouz MG, Galhoum AA, Gomaa NA, Abdel-Rehem SS, Atia AA, Vincent T, Guibal E (2015) Uranium extraction using magnetic nano-based particles of diethylenetriamine-functionalized chitosan: Equilibrium and kinetic studies. Chem Eng J 262:198–209. https://doi.org/10.1016/j.cej.2014.09.061
Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenker JL (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114(27):10834–10843. https://doi.org/10.1021/ja00053a020
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359(6397):710–712. https://doi.org/10.1038/359710a0
Wang Y, Zhang Y, Li Q, Li Y, Cao L, Li W (2020) Amidoximated cellulose fiber membrane for uranium extraction from simulated seawater. Carbohyd Polym 245:116627. https://doi.org/10.1016/j.carbpol.2020.116627
Salama A, Aljohani HA, Shoueir KR (2018) Oxidized cellulose reinforced silica gel: New hybrid for dye adsorption. Mater Lett 230:293–296. https://doi.org/10.1016/j.matlet.2018.07.131
Iftekhar S, Srivastava V, Sillanpää M (2017) Enrichment of lanthanides in aqueous system by cellulose based silica nanocomposite. Chem Eng J 320:151–159. https://doi.org/10.1016/j.cej.2017.03.051
Gunathilake C, Dassanayake RS, Abidi N, Jaroniec M (2016) Amidoxime-functionalized microcrystalline cellulose–mesoporous silica composites for carbon dioxide sorption at elevated temperatures. J Mater Chem A 4(13):4808–4819. https://doi.org/10.1039/C6TA00261G
Fan L, Huang G, Yang S, Xie Y, Liu W, Shi J (2021) Preparation of phosphate-functionalized biopolymer/graphene oxide gels for enhanced selective adsorption of U(VI) from aqueous solution. J Radioanal Nucl Chem 329(2):555–564. https://doi.org/10.1007/s10967-021-07723-x
Huynh J, Palacio R, Safizadeh F, Lefèvre G, Descostes M, Lilian E, Guignard N, Rousseau J, Royer S, Tertre E, Batonneau-Gener I (2017) Uranium in water adsorption over NH2 functionalized ordered silica. ACS Appl Mater Interf 9. https://doi.org/10.1021/acsami.6b16158
Zheng H, Zhou L, Liu Z, Le Z, Ouyang J, Huang G, Shehzad H (2019) Functionalization of mesoporous Fe3O4@SiO2 nanospheres for highly efficient U(VI) adsorption. Microporous Mesoporous Mater 279:316–322. https://doi.org/10.1016/j.micromeso.2018.12.038
Dashtian K, Zare-Dorabei R (2017) Synthesis and characterization of functionalized mesoprous SBA-15 decorated with Fe3O4 nanoparticles for removal of Ce(III) ions from aqueous solution: ICP–OES detection and central composite design optimization. J Colloid Interface Sci 494:114–123. https://doi.org/10.1016/j.jcis.2017.01.072
Yang S, Huang Y, Huang G, Peng W, Guo C, Shi J (2020) Preparation of amidoxime-functionalized biopolymer/graphene oxide gels and their application in selective adsorption separation of U(VI) from aqueous solution. J Radioanal Nucl Chem 324(2):847–855. https://doi.org/10.1007/s10967-020-07101-z
Zhuang S, Cheng R, Kang M, Wang J (2018) Kinetic and equilibrium of U(VI) adsorption onto magnetic amidoxime-functionalized chitosan beads. J Clean Prod 188:655–661. https://doi.org/10.1016/j.jclepro.2018.04.047
Sing KSW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619. https://doi.org/10.1351/pac198557040603
Zhao Y, Li J, Zhao L, Zhang S, Huang Y, Wu X, Wang X (2014) Synthesis of amidoxime-functionalized Fe3O4@SiO2 core–shell magnetic microspheres for highly efficient sorption of U(VI). Chem Eng J 235:275–283. https://doi.org/10.1016/j.cej.2013.09.034
Ahmad M, Wang J, Yang Z, Zhang Q, Zhang B (2020) Ultrasonic-assisted preparation of amidoxime functionalized silica framework via oil-water emulsion method for selective uranium adsorption. Chem Eng J 389:124441. https://doi.org/10.1016/j.cej.2020.124441
Ji G, Zhu G, Wang X, Wei Y, Yuan J, Gao C (2017) Preparation of amidoxime functionalized SBA-15 with platelet shape and adsorption property of U(VI). Sep Purif Technol 174:455–465. https://doi.org/10.1016/j.seppur.2016.10.048
Zhao Y, Li J, Zhang S, Wang X (2014) Amidoxime-functionalized magnetic mesoporous silica for selective sorption of U(vi). RSC Adv 4(62):32710–32717. https://doi.org/10.1039/C4RA05128A
Dai Y, Jin J, Zhou L, Li T, Li Z, Liu Z, Huang G, Adesina AA (2017) Preparation of hollow SiO2 microspheres functionalized with amidoxime groups for highly efficient adsorption of U(VI) from aqueous solution. J Radioanal Nucl Chem 311(3):2029–2037. https://doi.org/10.1007/s10967-016-5128-3
Yin X, Bai J, Tian W, Li S, Wang J, Wu X, Wang Y, Fan F, Huang Q, Qin Z (2017) Uranium sorption from saline lake brine by amidoximated silica. J Radioanal Nucl Chem 313(1):113–121. https://doi.org/10.1007/s10967-017-5283-1
Kouraim MN, Hagag M, Ali AH (2020) Adsorption of uranium from its aqueous solutions using activated cellulose and silica grafted cellulose. Radiochim Acta 108(4):261–271. https://doi.org/10.1515/ract-2019-3149
Fan Q-h, Li P, Chen Y-f, Wu W-s (2011) Preparation and application of attapulgite/iron oxide magnetic composites for the removal of U(VI) from aqueous solution. J Hazard Mater 192(3):1851–1859. https://doi.org/10.1016/j.jhazmat.2011.07.022
Li D, Egodawatte S, Kaplan DI, Larsen SC, Serkiz SM, Seaman JC, Scheckel KG, Lin J, Pan Y (2017) Sequestration of U(VI) from acidic, alkaline, and high ionic-strength aqueous media by functionalized magnetic mesoporous silica nanoparticles: capacity and binding mechanisms. Environ Sci Technol 51(24):14330–14341. https://doi.org/10.1021/acs.est.7b03778
Tang N, Liang J, Niu C, Wang H, Luo Y, Xing W, Ye S, Liang C, Guo H, Guo J, Zhang Y, Zeng G (2020) Amidoxime-based materials for uranium recovery and removal. Journal of Materials Chemistry A 8(16):7588–7625. https://doi.org/10.1039/C9TA14082D
Acknowledgements
The present work was partially supported by the national science foundation of China (21866005), Jiangxi Key plan of research and development (20192BBH80011).
Funding
Innovative Research Group Project of the National Natural Science Foundation of China,21866005,wenbin Liu
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, W., Huang, Y., Huang, G. et al. Eco-friendly and low-cost amidoxime-functionalized microcrystalline cellulose/mesoporous silica composite for the selective adsorption of U(VI) from aqueous solution. J Radioanal Nucl Chem 331, 2055–2068 (2022). https://doi.org/10.1007/s10967-022-08261-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08261-w