Abstract
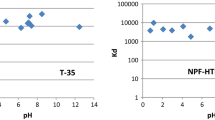
The paper presents the results of the cesium sorption from seawater with commercially available sorbents (Anfezh, Niket, Uniket, FSS, FD-M, Axionit RCs, Termoxid 35, Termoxid 3A, NKF-C) under the batch and flow conditions, including the distribution coefficients of 137Cs, the dynamic exchange capacity and the total dynamic exchange capacity of the sorbents for cesium as well as the output curves of cesium sorption at different flow rates of the seawater. The data obtained makes it possible to select the most effective sorbents for concentrating and analyzing radiocesium in seawater. Some of the sorbents are effective for 137Cs sorption from seawater. The maximum distribution coefficients of 137Cs under the batch conditions are more than 6 × 104 mL g−1 for the sorbents Anfezh and FD-M. The maximum values of the total dynamic exchange capacity in the process of the non-radioactive cesium sorption for the Uniket and Niket sorbents are 77.7 and 88.8 mg g−1, respectively.


Similar content being viewed by others
References
Kotelyanets EA, Gurov KI, Tikhonova EA et al (2019) Pollutants in bottom sediments in the Balaklava Bay (the Black Sea). Phys Oceanogr 26:414–424. https://doi.org/10.22449/1573-160X-2019-5-414-424
Buesseler KO, Livingston HD (1997) Time-series profiles of 134CS, 137CS, and 90SR in the Black Sea. In: Özsoy E, Mikaelyan A (eds) Sensitivity to Change: Black Sea, Baltic Sea, and the North Sea. Springer Netherlands, Dordrecht, pp 239–251. https://doi.org/10.1007/978-94-011-5758-2_19
Egorov VN, Povinec PP, Polikarpov GG et al (1999) 90Sr and 137Cs in the Black Sea after the Chernobyl NPP accident: inventories, balance and tracer applications. J Environ Radioact 43:137–155. https://doi.org/10.1016/S0265-931X(98)00088-5
Staneva JV, Buesseler KO, Stanev EV, Livingston HD (1999) The application of radiotracers to a Black Sea circulation study: validation of numerical simulations against observed weapons testing and Chernobyl 137Cs data. J Geophys Res Ocean 104:11099–11114. https://doi.org/10.1029/1998JC900121
Gulin SB, Mirzoyeva NYu, Egorov VN et al (2013) Secondary radioactive contamination of the Black Sea after Chernobyl accident: current levels, pathways, and trends. J Environ Radioact 124:50–56. https://doi.org/10.1016/j.jenvrad.2013.04.001
Gulin SB, Egorov VN, Duka MS et al (2015) Deep-water profiling of 137Cs and 90Sr in the Black Sea: further insight into the dynamics of the post-Chernobyl radioactive contamination. J Radioanal Nucl Chem 304:779–783. https://doi.org/10.1007/s10967-014-3848-9
Delfanti R, Özsoy E, Kaberi H et al (2014) Evolution and fluxes of 137Cs in the Black Sea/Turkish Straits System/North Aegean Sea. J Mar Syst 135:117–123. https://doi.org/10.1016/j.jmarsys.2013.01.006
Lehto J, Hou X (2011) Chemistry and analysis of radionuclides: laboratory techniques and methodology. Wiley, New York
Hirose K, Aoyama M, Igarashi Y, Komura K (2008) Improvement of 137Cs analysis in small volume seawater samples using the Ogoya underground facility. J Radioanal Nucl Chem 276:795–798. https://doi.org/10.1007/s10967-008-0634-6
Roger S, Wilson T (1974) A simple and precise analytical method for determining cesium-137 in seawater. ICES J Mar Sci 36:87–89. https://doi.org/10.1093/icesjms/36.1.87
Vincent T, Vincent C, Guibal E (2015) Immobilization of metal hexacyanoferrate ion-exchangers for the synthesis of metal ion sorbents—a mini-review. Mol 20:20582–20613. https://doi.org/10.3390/molecules201119718
Goto S, Umino S, Amakai W et al (2016) Impregnation structure of cobalt ferrocyanide microparticles by the polymer chain grafted onto nylon fiber. J Nucl Sci Technol 53:1251–1255. https://doi.org/10.1080/00223131.2016.1143886
Watari K, Izawa M (1965) Separation of radiocesium by copper ferrocyanide-anion exchange resin. J Nucl Sci Technol 2:321–322. https://doi.org/10.3327/jnst.2.321
Watari K, Imai K, Izawa M (1967) Isolation of 137 Cs with copper ferrocyanide-anion exchange resin. J Nucl Sci Technol 4:190–194. https://doi.org/10.1080/18811248.1967.9732723
Mann DR, Casso SA (1984) In situ chemisorption of radiocesium from seawater. Mar Chem 14:307–318. https://doi.org/10.1016/0304-4203(84)90027-6
Terada K (1970) Silica gel as support for inorganic ion- exchangers for the determination of cesium-137 in natural waters. Talanta 17:955–963. https://doi.org/10.1016/0039-9140(70)80138-2
Bokor I, Sdraulig S, Jenkinson P et al (2016) Development and validation of an automated unit for the extraction of radiocaesium from seawater. J Environ Radioact 151:530–536. https://doi.org/10.1016/j.jenvrad.2015.08.015
Kosyakov VN, Veleshko AN, Veleshko IE (2006) Determination of 137Cs in seawater under the field conditions. Radiochem 48:589–592. https://doi.org/10.1134/S1066362206060099
Kosyakov VN, Veleshko IE, Yakovlev NG, Gorovoi LF (2004) Preparation, properties, and application of modified mikoton sorbents. Radiochem 46:385–390. https://doi.org/10.1023/B:RACH.0000039117.10307.d0
NIKET-the sorbent for extraction of cesium radionuclides from liquids. https://eksorb.com/en/technologies/sorbenty-dlya-zhro-aes/niket/. Accessed 27 July 2020
Semenishchev VS, Pyankov AA, Remez VP et al (2020) Study of physicochemical and sorption properties of nickel and iron hexacyanoferrates to cesium. Sorpt Chromatogr Process 20:54–63. https://doi.org/10.17308/sorpchrom.2020.20/2380
Leppänen A-P, Kasatkina N, Vaaramaa K et al (2013) Selected anthropogenic and natural radioisotopes in the Barents Sea and off the western coast of Svalbard. J Environ Radioact 126:196–208. https://doi.org/10.1016/j.jenvrad.2013.08.007
Remez VP, Sapozhnikov YuA (1996) The rapid determination of caesium radionuclides in water systems using composite sorbents. Appl Radiat Isot 47:885–886. https://doi.org/10.1016/S0969-8043(96)00080-2
Remez VP, Zheltonozhko EV, Sapozhnikov YA (1998) The experience of using ANFEZH sorbent for recovery of radioactive caesium from sea water. Radiat Prot Dosim 75:77–78. https://doi.org/10.1093/oxfordjournals.rpd.a032251
Remez VP, Zelenin VI, Smirnov AL et al (2009) Cellulose-inorganic sorbents in radiochemical analysis I. Promising sorbents for radiochemical analysis. Sorpt Chromatogr Process 9:627–632
Remez VP, Zelenin VI, Smirnov AL et al (2009) Cellulose-inorganic sorbents in radiochemical analysis II. Synthesis and properties of the ANFEZH® sorbent. Sorpt Chromatogr Process 9:739–744
Remez VP, Zelenin VI, Smirnov AL et al (2009) Cellulose-inorganic sorbents in radiochemical analysis III. The concentration of radiocesium by the ANFEZH® sorbent. Sorpt Chromatogr Process 9:783–787
Goryachev VA, Tananaev IG, Dergunova DP (2018) Extraction of 134,137Cs by cellulose-inorganic sorbent based on ferrocyanide iron-potassium “ANFEZH” from LRW containing seawater. Radiat Saf Matters 89:12–18
Johnson BE, Santschi PH, Addleman RS et al (2011) Optimization and evaluation of mixed-bed chemisorbents for extracting fission and activation products from marine and freshwaters. Anal Chim Acta 708:52–60. https://doi.org/10.1016/j.aca.2011.08.017
Bandong BB, Volpe AM, Esser BK, Bianchini GM (2001) Pre-concentration and measurement of low levels of gamma-ray emitting radioisotopes in coastal waters. Appl Radiat Isot 55:653–665. https://doi.org/10.1016/S0969-8043(01)00081-1
Nakanishi T, Aono T, Yamada M, Kusakabe M (2010) Temporal and spatial variations of 137Cs in the waters of a nuclear fuel reprocessing facility in Rokkasho, Aomori, Japan. J Radioanal Nucl Chem 283:831–838. https://doi.org/10.1007/s10967-009-0422-y
Dovhyi II, Kremenchutskii DA, Bezhin NA et al (2020) Distribution of 137Cs in the Surface Layer of the Black Sea in Summer, 2017. Phys Oceanogr 27:152–160. https://doi.org/10.22449/1573-160X-2020-2-152-160
Pekárek V, Veselý V (1972) Synthetic inorganic ion exchangers—II: salts of heteropolyacids, insoluble ferrocyanides, synthetic aluminosilicates, and miscellaneous exchangers. Talanta 19:1245–1283. https://doi.org/10.1016/0039-9140(72)80124-3
Vincent T, Vincent C, Barré Y et al (2014) Immobilization of metal hexacyanoferrates in chitin beads for cesium sorption: synthesis and characterization. J Mater Chem A 2:10007–10021. https://doi.org/10.1039/C4TA01128G
Nilchi A, Malek B, Maragheh MG, Khanchi A (2003) Exchange properties of cyanide complexes. J Radioanal Nucl Chem 258:457–462. https://doi.org/10.1023/B:JRNC.0000011738.46843.ff
Bondar Y, Kuzenko S, Han D-H, Cho H-K (2014) Development of novel nanocomposite adsorbent based on potassium nickel hexacyanoferrate-loaded polypropylene fabric. Nanoscale Res Lett 9:180. https://doi.org/10.1186/1556-276X-9-180
Ishihara R, Fujiwara K, Harayama T et al (2011) Removal of cesium using cobalt-ferrocyanide-impregnated polymer-chain-grafted fibers. J Nucl Sci Technol 48:1281–1284. https://doi.org/10.1080/18811248.2011.9711817
Okamura Y, Fujiwara K, Ishihara R et al (2014) Cesium removal in freshwater using potassium cobalt hexacyanoferrate-impregnated fibers. Radiat Phys Chem 94:119–122. https://doi.org/10.1016/j.radphyschem.2013.04.011
Tokar’ E, Zemskova L, Tutov M et al (2020) Development and practical evaluation of the scheme for 137Cs concentrating from seawater using chitosan and mixed ferrocyanides of Zn-K and Ni-K. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-020-07248-9
Egorin A, Tokar E, Zemskova L et al (2017) Chitosan-ferrocyanide sorbents for concentrating Cs-137 from seawater. Sep Sci Technol 52:1983–1991. https://doi.org/10.1080/01496395.2017.1321669
Egorin AM, Palamarchuk MS, Tokar’ EA et al (2017) Sorption of 137Cs from seawater onto resorcinol–formaldehyde resin. Radiochem 59:160–165. https://doi.org/10.1134/S1066362217020084
Egorin A, Palamarchuk M, Tokar E et al (2017) Concentrating cesium-137 from seawater using resorcinol-formaldehyde resin for radioecological monitoring. Radiochim Acta 105:121–127. https://doi.org/10.1515/ract-2015-2522
Milyutin VV, Zelenin PG, Kozlov PV et al (2019) Sorption of cesium from alkaline solutions onto resorcinol-formaldehyde sorbents. Radiochem 61:714–718. https://doi.org/10.1134/S1066362219060122
Matel L, Dulanska S, Silikova V (2018) Composite sorbents for radionuclide separation. In: XXXIX Days of Radiation Protection. Proceedings of Presentations and Posters. Slovakia: Slovenska zdravotnicka univerzita., p 578
Nada AMA, Moussa WM, El-Mongy SA, El-Sayed ESA (2009) Physicochemical studies of cation ion exchange wood pulp. Australian J Basic Appl Sci 3:9–16
Sharygin LM, Muromskii AY (2000) New inorganic sorbent for ion-selective purification of liquid radioactive wastes. At Energy 89:658–662. https://doi.org/10.1023/A:1011303609753
Sharygin LM, Muromskii AYu (2004) Inorganic sorbent for selective treatment of liquid radioactive wastes. Radiochem 46:185–189. https://doi.org/10.1023/B:RACH.0000024949.11280.15
Voronina AV, Noskova AYu, Semenishchev VS, Gupta DK (2020) Decontamination of seawater from 137Cs and 90Sr radionuclides using inorganic sorbents. J Environ Radioact 217:106210. https://doi.org/10.1016/j.jenvrad.2020.106210
Yurmanov VA, Belous VN, Vasina VN, Yurmanov EV (2010) Chemistry and corrosion issues in supercritical water reactors. In: Proceedings of the nuclear plant chemistry conference (NPC 2010). Quebec City, Canada, Canadian Nuclear Society, Toronto, pp 3–8
Breier CF, Pike SM, Sebesta F et al (2016) New applications of KNiFC-PAN resin for broad-scale monitoring of radiocesium following the Fukushima Dai-ichi nuclear disaster. J Radioanal Nucl Chem 307:2193–2200. https://doi.org/10.1007/s10967-015-4421-x
Pike SM, Buesseler KO, Breier CF et al (2013) Extraction of cesium in seawater off Japan using AMP-PAN resin and quantification via gamma spectroscopy and inductively coupled mass spectrometry. J Radioanal Nucl Chem 296:369–374. https://doi.org/10.1007/s10967-012-2014-5
Dovhyi II, Bezhin NA, Kapranov SV, Lyapunov AY (2020) Lead sorption by extraction chromatographic resins on the base Di-(tert-butylcyclohexano)-18-crown-6 and its application for analysis of marine samples. J Radioanal Nucl Chem 324:1189–1201. https://doi.org/10.1007/s10967-020-07164-y
Buesseler K (2013) Cs from 20L using KNiFC-PAN resin. https://cmer.whoi.edu/recipe/cs-from-20l-using-knifc-pan-resin/. Accessed 27 July 2020
Buesseler K (2011) Cs from 20L using AMP-PAN resin columns. 2011. https://cmer.whoi.edu/recipe/cs-from-20l-using-amp-pan-resin-columns/. Accessed 27 July 2020
CS RESINS (Resins and Accessories). https://www.triskem-international.com/catalog/products/resins-and-accessories/cs-resins/bl,product,420,0. Accessed 16 December 2020
Acknowledgements
RFBR funded the reported study, Project Number 19-33-60007 (the “Perspective” competition), the state assignment of the Ministry of Science and Higher Education of the Russian Federation (the Oceanological processes” Topic No. 0827-2020-0003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bezhin, N.A., Dovhyi, I.I., Milyutin, V.V. et al. Study of sorbents for analysis of radiocesium in seawater samples by one-column method. J Radioanal Nucl Chem 327, 1095–1103 (2021). https://doi.org/10.1007/s10967-020-07588-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07588-6