Abstract
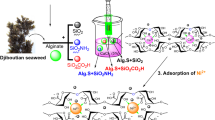
The syntheses of crown ether-type organic composite adsorbents embedded in high-porous silica beads for simultaneous recovery of lithium and uranium in seawater have been achieved and the adsorption behavior of lithium and uranium on the composite adsorbents has been examined in several types of original seawater in the wide temperature and pH ranges. As a result, the composite adsorbents composed of benzo-15-crown-5 (BC15) and benzo-18-crown-6 (BC18) showed the top-class maximum adsorption capacities for lithium [6.5 mg/g (BC15), 11 mg/g (BC18)] and uranium [12 mg/g (BC15), 4.2 mg/g (BC18)].









Similar content being viewed by others
References
Cheng X, Zhang R, Zhao C, Zhang Q (2017) Toward safe lithium metal anode in rechargeable batteries: a review. Chem Rev 117:10403–10473
Sawada A (2012) Current situation of lithium resource development in the world. Bull Soc Sea Water Sci Jpn 66:2–7
Putra AR, Tachibana Y, Tanaka M, Suzuki T (2018) Lithium isotope separation using displacement chromatography by cation exchange resin with high degree of cross-linkage. Fusion Eng Des 136:377–380
Hoshino T (2015) Innovative lithium recovery technique from seawater by using world-first dialysis with a lithium ionic superconductor. Desalination 359:59–63
Yoshizuka K (2012) Practical recovery of lithium from seawater. J Ion Exch 23:59–65
Isshiki K (2005) In: Fujinaga T, Sohrin Y, Isshiki K (eds) Ocean & lake chemistry, 1st edn. Kyoto University Press, Kyoto
Tachibana Y, Suzuki T, Nogami M, Nomura M, Kaneshiki T (2018) Selective lithium recovery from seawater using crown ether resins. J Ion Exch 29:90–96
Tachibana Y, Nair VV, Mohan S, Suzuki T (2019) Chromatographic fractionation of lithium isotope in aqueous solution using bifunctional ion exchange resin. Sep Sci Technol 1:1. https://doi.org/10.1080/01496395.2019.1607874 (in press)
Swain B (2017) Recovery and recycling of lithium: a review. Sep Purif Technol 172:388–403
Kurniawan YS, Sathuluri RR, Ohto K, Iwasaki W, Kawakita H, Morisada S, Miyazaki M, Jumina (2019) A rapid and efficient lithium-ion recovery from seawater with tripropyl-monoacetic acid calix[4]arene derivative employing droplet-based microreactor system. Sep Purif Technol 211:925–934
Hiraoka M (1982) Crown compounds: their characteristics and applications, 1st edn. Kodansha, Tokyo
Bond AH, Dietz ML, Chiarizia R (2000) Incorporating size selectivity into synergistic solvent extraction: a review of crown ether-containing systems. Ind Eng Chem Res 39:3442–3464
Yan F, Pei H, Pei Y, Li T, Li J, He B, Cheng Y, Cui Z, Guo D, Cui J (2015) Preparation and characterization of polysulfone-graft-4′-aminobenzo-15-crown-5-ether for lithium isotope separation. Ind Eng Chem Res 54:3473–3479
Abney CW, Mayes RT, Saito T, Dai S (2017) Materials for the recovery of uranium from seawater. Chem Rev 117:13935–14013
Lindner H, Schneider E (2015) Review of cost estimates for uranium recovery from seawater. Energy Econ 49:9–22
Yamazaki Y, Tachibana Y, Kaneshiki T, Nomura M, Suzuki T (2015) Adsorption behavior of uranium ion using novel phenol-type resins in contaminated water containing seawater. Prog Nucl Energy 82:74–79
Grenthe I, Fuger J, Konings RJM, Lemire RJ, Muller AB, Cregu CNT, Wanner H (2004) In: Wanner H, Forest I (eds) Chemical thermodynamics of uranium, 1st edn. OECD Pubilications, Paris
Ma S, Huang L, Ma L, Shim Y, Islam SM, Wang P, Zhao LD, Wang S, Sun G, Yang X, Kanatzidis MG (2015) Efficient uranium capture by polysulfide/layered double hydroxide composites. J Am Chem Soc 137:3670–3677
Kavakli PA, Seko N, Tamada M, Güven O (2005) A Highly efficient chelating polymer for the adsorption of uranyl and vanadyl ions at low concentrations. Adsorption 10:309–315
Omichi H, Katakai A, Sugo T, Okamoto J, Katoh S, Sakane K, Sugasaka K, Itagaki T (1987) Effect of shape and size of amidoxime-group-containing adsorbent on the recovery of uranium from seawater. Sep Sci Technol 22:1313–1325
Oyola Y, Dai S (2016) High surface-area amidoxime-based polymer fibers co-grafted with various acid monomers yielding increased adsorption capacity for the extraction of uranium from seawater. Dalton Trans 45:8824–8834
Brown S, Yue Y, Kuo LJ, Mehio N, Li M, Gill G, Tsouris C, Mayes RT, Saito T, Dai S (2016) Uranium adsorbent fibers prepared by atom-transfer radical polymerization (ATRP) from poly(vinyl chloride)-co-chlorinated poly(vinyl chloride) (PVC-coCPVC) Fiber. Ind Eng Chem Res 55:4139–4148
Ismail AF, Yim MS (2015) Investigation of activated carbon adsorbent electrode for electrosorption-based uranium extraction from seawater. Nucl Eng Technol 47:579–587
Feng Y, Jiang H, Li S, Wang J, Jing X, Wang Y, Chen M (2013) Metal-organic frameworks HKUST-1 for liquid-phase adsorption of uranium. Colloids Surf A 431:87–92
Chouyyok W, Pittman JW, Warner MG, Nell KM, Clubb DC, Gill GA, Addleman RS (2016) Surface functionalized nanostructured ceramic sorbents for the effective collection and recovery of uranium from seawater. Dalton Trans 45:11312–11325
Kou S, Yang Z, Sun F (2017) Protein hydrogel microbeads for selective uranium mining from seawater. ACS Appl Mater Interfaces 9:2035–2039
Nakajima A, Sakaguchi T (1990) Recovery of uranium by tannin immobilized on matrices which have amino group. J Chem Technol Biotechnol 47:31–38
Tachibana Y, Nogami M, Nomura M, Suzuki T (2016) Simultaneous removal of various iodine species in aqueous solutions of high salt concentrations using novel functional adsorbents. J Radioanal Nucl Chem 307:1911–1918
Products list of ion exchange and chelating resins, Mitsubishi Chemical, Co., Ltd. https://www.diaion.com/en/products/index.html. Accessed 8 Jun 2019
Tachibana Y, Putra AR, Hashimoto S, Suzuki T, Tanaka M (2018) Lithium isotope fractionation in weak basic solution using cation exchange chromatography. J Ion Exch 29:41–47
Ohta A (2006) Experimental and theoretical studies of REE partitioning between Fe hydroxide and Mn oxide and seawater. Geochemistry 40:13–30
Choi SH, Nho YC (2000) Adsorption of UO2 2+ by polyethylene adsorbents with amidoxime, carboxyl, and amidoxime/carboxyl group. Radiat Phys Chem 57:187–193
Zhang H, Liang H, Chen Q, Shen X (2013) Synthesis of a new ionic imprinted polymer for the extraction of uranium from seawater. J Radioanal Nucl Chem 298:1705–1712
Tachibana Y, Suzuki T, Nogami M, Nomura M, Kaneshiki T (2018) Syntheses of tannic acid-type organic composite adsorbents for simultaneous removal of various types of radionuclides in seawater. J Radioanal Nucl Chem 318:429–437
Milonjić SK (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serb Chem Soc 72:1363–1367
Anastopoulos I, Kyzas GZ (2016) Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena? J Mol Liq 218:174–185
Rahmani-Sani A, Shan RR, Yan LG, Hosseini-Bandegharaei A (2017) Response to “letter to editor: minor correction to the thermodynamic calculation using the distribution constant by Shan et al. and Rahmani-Sani et al. J Hazard Mater 325:367–368
Ilaiyaraja P, Deb AKS, Ponraju D, Ali SM, Venkatraman B (2017) Surface engineering of PAMAM-SDB chelating resin with diglycolamic acid (DGA) functional group for efficient sorption of U(VI) and Th(IV) from aqueous medium. J Hazard Mater 328:1–11
Kikuchi E, Segawa K, Tada A, Imizu Y, Hattori H (2001) New catalytic chemistry, 2nd edn. Sankyo Shuppan, Tokyo
Fujii T (2017) CO2 dynamics of the atmosphere and surface seawater in coastal zone. J Jpn Assoc Hydrol Sci 47:107–118
Chitrakar R, Kanoh H, Miyai Y, Ooi K (2001) Recovery of lithium from seawater using manganese oxide adsorbent (H1.6Mn1.6O4) derived from Li1.6Mn1.6O4. Ind Eng Chem Res 40:2054–2058
Acknowledgements
This work was partially supported by JSPS KAKENHI Grant Number 16K18346 and the NIFS Collaboration Research Program (Grant Number: NIFS18KESA029). The authors would like to thank Mr. Toshitaka Kaneshiki and Dr. Masao Nomura (Laboratory for Advanced Nuclear Energy, Tokyo Institute of Technology) and Dr. Tastuya Suzuki (Nagaoka University of Technology) for fruitful discussion and technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tachibana, Y., Tanaka, M. & Nogami, M. Crown ether-type organic composite adsorbents embedded in high-porous silica beads for simultaneous recovery of lithium and uranium in seawater. J Radioanal Nucl Chem 322, 717–730 (2019). https://doi.org/10.1007/s10967-019-06792-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06792-3