Abstract
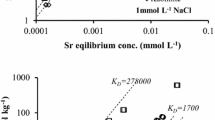
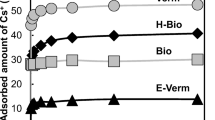
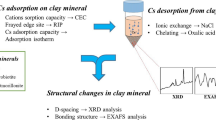
This study aimed to reveal local structures of strontium (Sr) and cesium (Cs) adsorbed on clay minerals (vermiculite and montmorillonite), which suggests stabilities of the adsorbed species of 90Sr and 137Cs in the environment. Adsorption experiments to determine solid-water distribution, XRD for interlayer distances of the clay minerals, and EXAFS for speciation of Sr and Cs were conducted. They showed that Sr and Cs formed outer- and inner-sphere complexes, respectively, in the interlayer, which explains the different degree of mobility of 90Sr and 137Cs in the environment and indicates larger contribution of electrostatic effect for Sr2+ than for Cs+.



Similar content being viewed by others
References
Choppin RG, Liljenzin J-O, Ryoberg J (2002) Radiochemistry and nuclear chemistry. Butterworth-Heinemann, Oxford
Vajda N, Kim CK (2010) Determination of radiostrontium isotopes: a review of analytical methodology. Appl Radiat Isot 68:2306–2326
Kryshev AI (2006) 90Sr in fish: a review of data and possible model approach. Sci Total Environ 370:182–189
Yoshida N, Takahashi Y (2012) Land-surface contamination by radionuclides from the Fukushima Daiichi nuclear power plant accident. Elements 8:201–206
Steinhauser G (2014) Fukushimas forgotten radionuclides: a review of the understudied radioactive emissions. Environ Sci Technol 48:4649–4663
Prand-Stritzko B, Steinhauser G (2018) Characteristics of radiocesium contaminations in mushrooms after the Fukushima nuclear accident: evaluation of the food monitoring data from March 2011 to March 2016. Environ Sci Pollut Res 25:2409–2416
Fan Q, Tanaka M, Tanaka K, Sakaguchi A, Takahashi Y (2014) An EXAFS study on the effects of natural organic matter and the expandability of clay minerals on cesium adsorption and mobility. Geochim Cosmochim Acta 135:49–65
Forsberg S, Strandmark M (2001) Migration and chemical availability of 137Cs and 90Sr in Swedish long-term experimental pastures. Water Air Soil Pollut 127:157–171
Forsberg S, Rosén K, Fernandez V, Juhan H (2000) Migration of 137Cs and 90Sr in undisturbed soil profiles under controlled and close-to-real conditions. J Environ Radioact 50:235–252
Ivanov YA, Lewyckyj N, Levchuk SE, Prister BS, Firsakova SK, Arkhipov NP, Arkhipov AN, Krainian SV, Alexakhini RM, Sandallsg J, Askbranth S (1997) Migration of 137Cs and 90Sr from chernobyl fallout in Ukrainian, Belarussian and Russian soils. J Environ Radioact 35:1–21
Lujaniene G, Plukis A, Kimtys E, Remeikis V, Jankünaite D, Ogorodnikov BI (2002) Study of 137Cs, 90 Sr, 239,240Pu, 238Pu and 241Am behavior in the chernobyl soil. J Radioanal Nucl Chem 251:59–68
Rosenberg BL, Ball JE, Shozugawa K, Korschinek G, Hori M, Nanba K, Johnson TE, Brandl A, Steinhauser G (2017) Radionuclide pollution inside the Fukushima Daiichi exclusion zone, part 1: depth profiles of radiocesium and strontium-90 in soil. Appl Geochem 85:201–208
Sahoo SK, Kavasi N, Sorimachi A, Arae H, Tokonami S, Mietelski JW, Lokas E, Yoshida S (2016) Strontium-90 activity concentration in soil samples from the exclusion zone of the Fukushima Daiichi nuclear power plant. Sci Rep 6:1–10
Sokolik GA, Ovsiannikova SV, Ivanova TG, Leinova SL (2004) Soil-plant transfer of plutonium and americium in contaminated regions of Belarus after the chernobyl catastrophe. Environ Int 30:939–947
Bostick BC, Vairavamurthy MA, Karthikeyan KG, Chorover J (2002) Cesium adsorption on clay minerals: an EXAFS spectroscopic investigation. Environ Sci Technol 36:2670–2676
Qin H, Yokoyama Y, Fan Q, Iwatani H, Tanaka K, Sakaguchi A, Kanai Y, Zhu J, Onda Y, Takahashi Y (2012) Investigation of cesium adsorption on soil and sediment samples from Fukushima prefecture by sequential extraction and EXAFS technique. Geochem J 46:297–302
Fuller AJ, Shaw S, Ward MB, Haigh SJ, Mosselmans JFW, Peacock CL, Stackhouse S, Dent AJ, Trivedi D, Burke IT (2015) Caesium incorporation and retention in illite interlayers. Appl Clay Sci 108:128–134
Zaunbrecher LK, Cygan RT, Elliott WC (2015) Molecular models of cesium and rubidium adsorption on weathered micaceous minerals. J Phys Chem A 119:5691–5700
Kerisit S, Okumura M, Rosso KM, Machida M (2016) Molecular simulation of cesium adsorption at the basal surface of phyllosilicate minerals. Clays Clay Miner 64:389–400
Sikalidis CA, Misaelides P, Alexiades CA (1988) Caesium selectivity and fixation by vermiculite in the presence of various competing cations. Environ Pollut 52:67–79
Lu N, Mason CFV (2001) Sorption–desorption behavior of strontium-85 onto montmorillonite and silica colloids. Appl Geochem 16:1653–1662
Sheha RR, Metwally E (2007) Equilibrium isotherm modeling of cesium adsorption onto magnetic materials. J Hazard Mater 143:354–361
Zabinsky SI, Rehr JJ, Ankudinov A, Albers RC, Eller MJ (1995) Multiple-scattering calculations of X-ray-absorption spectra. Phys Rev B 52:2995–3009
Ribár Β, Matković B, Sljukić M (1972) Die kristallstruktur von strontiumnitrat-tetrahydrat, Sr(NO3)2·4H2O. Z Krist 135:137–144
Araki T (1980) Crystal structure of a cesium alumosilicate, Cs(AlSi5O12). Z Krist 152:207–213
Vigdorchik AG, Malinovskii YA, Dryuchko AG (1990) Preparation and crystal structure of cesium-lanthanum nitrate Cs2La(NO3)5(H2O)2. Kristallografiya 35:1395–1398
James RO, Healy TX (1972) Adsorption of hydrolysable metal ions at the oxide-water interface: III. A thermodynamic model of adsorption. J Colloid Interface Sci 40:65–81
Li YH (1991) Distribution patterns of the elements in the oceans: a synthesis. Geochim Cosmochim Acta 55:3223–3240
Parks G (1967) Equilibrium concepts in natural water systems. American Chemical Society, Washington, DC
Hayes KF, Leckie JO (1987) Modeling ionic strength effects on cation adsorption at hydrous oxide solution interfaces. J Colloid Interface Sci 115:564–572
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. Wiley, Hoboken
Levy R, Shainberg I (1972) Calcium–magnesium exchange in montmorillonite and vermiculite. Clays Clay Miner 20:37–46
O’Day PA, Rehr JJ, Zabinsky SI, Brown GEJ (1994) Extended X-ray absorption fine structure (EXAFS) analysis of disorder and multiple scattering in complex crystalline solids. J Am Chem Soc 116:2938–2949
Fuller AJ, Shaw S, Peacock CL, Trivedi D, Burke IT (2016) EXAFS study of Sr sorption to illite, goethite, chlorite, and mixed sediment under hyperalkaline conditions. Langmuir 32:2937–2946
Chen C-C, Hayes KF (1999) X-ray absorption spectroscopy investigation of aqueous Co(II) and Sr(II) sorption at clay-water interfaces. Geochim Cosmochim Acta 63:3205–3215
Morodome S, Kawamura K (2011) In situ X-ray diffraction study of the swelling of montmorillonite as affected by exchangeable cations and temperature. Clays Clay Miner 59:165–175
Coughtrey PJ, Thorne MC (1984) Radionuclide distribution and transport in terrestrial and aquatic ecosystems. A.A. Balkema, Rotterdam
Arapis G, Petrayev E, Shagalova E, Zhukova O, Sokolik G, Ivanova T (1997) Effective migration velocity of 137Cs and 90Sr as a function of the type of soils in Belarus. J Environ Radioact 34:171–185
Grandia F, Sena C, Arcos D, Molinero J, Duro L, Bruno J (2011) Quantitative assessment of radionuclide retention in the Quaternary sediments/granite interface of the Fennoscandian shield (Sweden). Appl Geochem 26:679–687
Rosén K, Öborn I, Lönsjö H (1999) Migration of radiocaesium in Swedish soil profiles after the Chernobyl accident, 1987–1995. J Environ Radioact 46:45–66
Takahashi Y, Fan Q, Suga H, Tanaka K, Sakaguchi A, Takeichi Y, Ono K, Mase K, Kato K, Kanivets VV (2017) Comparison of Solid–Water partitions of radiocesium in river waters in Fukushima and chernobyl Areas. Sci Rep 7:1–11
Acknowledgements
This work was supported by the Reimei Research Promotion project (Japan Atomic Energy Agency) and JSPS KAKENHI Grant Numbers 17H04582, 16H04073, 16K13911, 16K12627, 15H02149, and 17H06458. This work has been performed with the approval of SPring-8/JASRI (Proposal Nos. 2015A0118, 2015B0118, and 2016A0118) and KEK (Proposal No. 2014G058). AY conducted experiments and wrote the manuscript. MT, YK, and YT discussed the results and contributed to the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yamaguchi, A., Tanaka, M., Kurihara, Y. et al. Local structure of strontium adsorbed on 2:1 clay minerals and its comparison with cesium by XAFS in terms of migration of their radioisotopes in the environment. J Radioanal Nucl Chem 317, 545–551 (2018). https://doi.org/10.1007/s10967-018-5895-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5895-0