Abstract
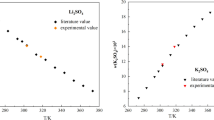
The phase equilibrium data were determined for the ternary systems potassium sulfate + polyethylene glycol (PEG6000/10,000) + water at 288.2, 298.2 and 308.2 K. Only solid–liquid equilibrium exists for the ternary system K2SO4 + PEG6000/10,000 + H2O at 288.2 and 298.2 K, the solubility, density, and refractive index were measured by using the isothermal dissolution equilibrium method. The solid–liquid equilibrium and liquid–liquid equilibrium exist simultaneously for the ternary system K2SO4 + PEG6000/10,000 + H2O at 308.2 K, the corresponding compositions of binodal curve and tie-line were obtained experimentally by using the turbidimetric method. Results show that the solubility of K2SO4 decreases as the content of PEG increases in the PEG–H2O mixed solvents. Meanwhile, as the molar mass of PEG increased, the liquid–liquid equilibrium formed more easily and the liquid–liquid equilibrium area was larger. In addition, the modified Pitzer model was used to calculate the solid–liquid equilibrium data for systems K2SO4 + PEG6000/10,000 + H2O at 288.2, 298.2 and 308.2 K.





Similar content being viewed by others
References
Silva, L.H.M., Silva, M.C.H., Júnior, A.J., Martins, J.P., Coimbra, J.S.R., Minim, L.A.: Hydrophobic effect on the partitioning of [Fe(CN)5(NO)]2– and [Fe(CN)6]3– anions in aqueous two-phase systems formed by triblock copolymers and phosphate salts. Sep. Purif. Technol. 60(1), 103–112 (2008)
Wang, P., Zhang, F., Li, P., Sun, T., Pan, Y.J., Zhang, Y.Q.: Partitioning performance of molybdenum in poly (ethyleneglycol) + sodium sulfate + water aqueous two-phase systems. J. Mol. Liq. 260, 211–217 (2018)
Taboada, M.E., Graber, T.A., Asenjo, J.A., Andrews, B.A.: Drowning-out crystallisation of sodium sulphate using aqueous two-phase systems. J. Chromatogr. B: Biomed. Sci. Appl. 743(1), 101–105 (2000)
Taboada, M.E., Palma, P.A., Graber, T.A.: Crystallization of potassium sulfate by cooling and salting-out using 1-propanol in a calorimetric reactor. Cryst. Res. Technol. 38(1), 21–29 (2003)
Jamehbozorg, B., Sadeghi, R.: Extractions of alkaloids codeine and caffeine with [Bmim][BF4]/carbohydrate aqueous biphasic systems as a novel class of liquid-liquid extraction systems. J. Chem. Eng. Data 64(3), 916–925 (2019)
Clavijo, V., Torres-Acosta, M.A., Vives-Flórez, M.J., Rito-Palomares, M.: Aqueous two-phase systems for the recovery and purification of phage therapy products: Recovery of salmonella bacteriophage ϕSan23 as a case study. Sep. Purif. Technol. 211, 322–329 (2019)
Silva, C.A.S., Coimbra, J.S.R., Rojas, E.E.G., Minim, L.A., Silva, L.H.M.: Partitioning of caseinomacropeptide in aqueous two-phase systems. J. Chromatogr. B 858(1–2), 205–210 (2007)
Rosa, P.A.J., Azevedo, A.M., Aires-Barros, M.R.: Application of central composite design to the optimisation of aqueous two-phase extraction of human antibodies. J. Chromatogr. A. 1141(1), 50–60 (2007)
Braas, G.M.F., Walker, S.G., Lyddiatt, A.: Recovery in aqueous two-phase systems of nanoparticulates applied as surrogate mimics for viral gene therapy vectors. J. Chromatogr. B: Biomed. Sci. Appl. 743(1–2), 409–419 (2000)
Graber, T.A., Gálvez, M.E., Galleguillos, H.R., Álvarez-Benedí, J.: Liquid–liquid equilibrium of the aqueous two-phase system water + PEG 4000 + lithium sulfate at different temperatures. experimental determination and correlation. J. Chem. Eng. Data 49(6), 1661–1664 (2004)
Wysoczanska, K., Do, H.T., Held, C., Sadowski, G., Macedo, E.A.: Effect of different organic salts on amino acids partition behaviour in PEG–salt ATPS. Fluid Phase Equilib. 456, 84–91 (2018)
Wysoczanska, K., Macedo, E.A.: Influence of the molecular weight of PEG on the polymer salt phase diagrams of aqueous two-phase systems. J. Chem. Eng. Data 61(12), 4229–4235 (2016)
Oliveira, A.C., Sosa, F.H.B., Costa, M.C., Filho, E.S.M., Ceriani, R.: Study of liquid–liquid equilibria in aqueous two-phase systems formed by poly(ethylene glycol) (PEG) and sodium thiosulfate pentahydrate (Na2S2O3·5H2O) at different temperatures. Fluid Phase Equilib. 476, 118–125 (2018)
Yu, X.D., Wang, L., Li, M.L., Huang, Q., Zeng, Y., Lan, Z.: Phase equilibria of CsCl–polyethylene glycol (PEG)–H2O at 298.15 K: effect of different polymer molar masses (PEG1000/4000/6000). J. Chem. Thermodyn. 135, 45–54 (2019)
Huang, Q., Wang, L., Li, M.L., Hu, P.P., Yu, X.D., Deng, H., Zeng, Y.: Measurements and simulation of the polyethylene glycol 1000–water–KCl ternary system at 288.2, 298.2, and 308.2 K. J. Chem. Eng. Jpn. 52(4), 325–332 (2019)
Yu, X.D., Huang, Q., Wang, L., Li, M.L., Zheng, H., Zeng, Y.: Measurements and simulation for ternary system KCl–PEG4000–H2O at T = 288, 298 and 308 K. CIESC J. 70(3), 830–839 (2019)
Govindarajan, R., Perumalsamy, M.: Phase equilibrium of PEG2000 + triammonium citrate + water system relating PEG molecular weight, cation, anion with effective excluded volume, Gibbs free energy of hydration, size of cation, and type of anion at (298.15, 308.15, and 318.15) K. J. Chem. Eng. Data 58, 2952–2958 (2013)
Urréjola, S., Sanchez, A., Hervello, M.F.: Solubilities of sodium, potassium, and copper(II) sulfates in ethanol–water solutions. J. Chem. Eng. Data 56(5), 2687–2691 (2011)
Jimenez, Y.P., Taboada, M.E., Galleguillos, H.R.: Solid–liquid equilibrium of K2SO4 in solvent mixtures at different temperatures. Fluid Phase Equilib. 284, 114–117 (2009)
Taboada, M.E., Véliz, D.M., Galleguillos, H.R., Graber, T.A.: Solubilities, densities, viscosities, electrical conductivities, and refractive indices of saturated solutions of potassium sulfate in water + 1-propanol at 298.15, 308.15, and 318.15 K. J. Chem. Eng. Data 47(5), 1193–1196 (2002)
Jimenez, Y.P., Taboada, M.E., Flores, E.K., Galleguillos, H.R.: Density, viscosity, and electrical conductivity in the potassium sulfate + water + 1-propanol system at different temperatures. J. Chem. Eng. Data 54(6), 1932–1934 (2009)
Jimenez, Y.P., Galleguillos, H.R.: (Liquid + liquid) equilibrium of (NaNO3 + PEG4000 + H2O) ternary system at different temperatures. J. Chem. Thermodyn. 43, 1573–1578 (2011)
Fosbl, P.L., Thomsen, K., Stenby, E.H.: Reverse Schreinemakers method for experimental analysis of mixed-solvent electrolyte systems. J. Solution Chem. 38(1), 1–14 (2008)
Lv, P., Zhong, Y., Meng, R.Y., Sun, B., Song, P.S.: Weighing titration analysis of the sulfate content. J. Salt Lake Res. 23(3), 5–13 (2015)
Cheluget, E.L., Gelinas, S., Vera, J.H., Weber, M.E.: Liquid–liquid equilibrium of aqueous mixtures of poly(propylene glycol) with NaCl. J. Chem. Eng. Data 39(1), 127–130 (1994)
Mistry, S.L., Kaul, A., Merchuk, J.C., Asenjo, J.A.: Mathematical modelling and computer simulation of aqueous two-phase continuous protein extraction. J. Chromatogr. A. 741(2), 151–163 (1996)
Hu, M.C., Zhai, Q.G., Jiang, Y.C., Jin, L.H., Liu, Z.H.: Liquid–liquid and liquid–liquid–solid equilibrium in PEG + Cs2SO4 + H2O. J. Chem. Eng. Data 49(5), 1440–1443 (2004)
Jayapal, M., Regupathi, I., Murugesan, T.: Liquid–liquid equilibrium of poly(ethylene glycol) 2000 + potassium citrate + water at (25, 35, and 45) °C. J. Chem. Eng. Data 52(1), 56–59 (2007)
Wu, Y.T., Lin, D.Q., Zhu, Z.Q., Mei, L.H.: Prediction of liquid–liquid equilibria of polymer–salt aqueous two-phase systems by a modified Pitzer’s virial equation. Fluid Phase Equilib. 124, 67–79 (1996)
Krevelen, D.W.V., Nijehuis, K.T.: Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th edn. Elsevier, Amsterdam (2009)
Greenberg, J.P., Møller, N.: The prediction of mineral solubilities in natural waters: a chemical equilibrium model for the Na–K–Ca–Cl–SO4–H2O system to high concentration from 0 to 250 ℃. Geochim. Cosmochim. Acta 53, 2503–2518 (1989)
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (U1507111).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, H., Chen, S., Wang, L. et al. Phase Equilibria of the Ternary Systems Potassium Sulfate + Polyethylene Glycol (PEG6000/10,000) + Water at 288.2, 298.2 and 308.2 K: Experimental Determination and Correlation. J Solution Chem 49, 1154–1169 (2020). https://doi.org/10.1007/s10953-020-01017-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-01017-8