Abstract
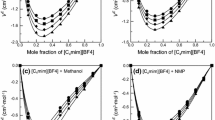
Densities, viscosities and refractive indices were determined for two ionic liquid mixtures formed by the 1-butyl-3-methylimidazolium glutamic acid salt ([Bmim][Glu]) or the 1-butyl-3-methylimidazolium glycine acid salt ([Bmim][Gly]), respectively, with methanol over the mole fraction from 0.1 to 0.9 and at temperatures ranging from 298.15 K to 313.15 K at intervals of 5 K and at atmospheric pressure. Excess molar volumes, viscosity deviations and refractive index deviations have been calculated from the experimental data and fitted to a Redlich–Kister polynomial function. The results have been interpreted in terms of ion-dipole interactions, and structural factors of the ionic liquid and alcohol molecular liquids.








Similar content being viewed by others
References
Krishan, L., Neelima, T., Gyan, P.D.: Densities, viscosities, and refractive indices of binary liquid mixtures of hexane, decane, hexadecane, and squalane with benzene at 298.15 K. J. Chem. Eng. Data 45, 961–964 (2000)
Carmichael, A.J., Seddon, K.R.: Polarity study of some 1-alkyl-3-methylimidazolium ambient-temperature ionic liquids with the solvatochromic dye, Nile red. J. Phys. Org. Chem. 13, 591–595 (2000)
Law, G., Watson, P.R.: Surface orientation in ionic liquids. Chem. Phys. Lett. 345, 1–4 (2001)
Seddon, K.R., Stark, A., Torres, M.J.: Influence of chloride, water and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 72, 2275–2287 (2000)
Rodriguez, H., Brennecke, J.F.: Temperature and composition dependence of the density and viscosity of binary mixtures of water + ionic liquid. J. Chem. Eng. Data 51, 2145–2155 (2006)
Gonzalez, E.J., Alonso, L., Dominguez, A.: Physical properties of binary mixtures of the ionic liquid 1-methyl-3-octylimidazolium chloride with methanol, ethanol, and 1-propanol at T=(298.15,313.15, and 328.15) K and at p=0.1 MPa. J. Chem. Eng. Data 51, 1446–1452 (2006)
Gomez, E., Gonzalez, B., Calvar, N., Tojo, E., Dominguez, A.: Physical properties of pure 1-ethyl-3-methylimidazolium ethylsulfate and its binary mixtures with ethanol and water at several temperatures. J. Chem. Eng. Data 51, 2096–2102 (2006)
Gao, H., Yu, Z., Wang, H.: Densities and volumetric properties of binary mixtures of amino acid ionic liquid [Bmim][Glu] or [Bmim][Gly] with benzylalcohol at T=(298.15 to 313.15) K. J. Chem. Thermodyn. 42, 640–645 (2010)
Fukumoto, K., Yoshizawa, M., Ohno, H.: Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 127, 2398–2399 (2005)
Ying, W., Qing-Guo, Z., Yang, L., Xiaorui, L., Suoyuan, L., Zhenhui, K.: Physicochemical property estimation of an ionic liquid based on glutamic acid–BMIGlu. J. Chem. Eng. Data 55, 2616–2619 (2010)
Tejraj, M.A., Bindu, G.: Densities, viscosities, and refractive indices of the binary mixtures of bis (2-methoxyethyl) ether with 1-propanol, 1-butanol, 2-methyl-1-propanol, and 2-methyl-2-propanol. J. Chem. Eng. Data 39, 865–867 (1994)
Tu, C., Lee, S., Peng, I.: Excess volumes and viscosities of binary mixtures of aliphatic alcohols (C1–C4) with nitromethane. J. Chem. Eng. Data 46, 151–155 (2001)
Gomez, A.C., Solimo, H.N.: Density, viscosity, excess molar volume, viscosity deviation, and their correlations for formamide + three alkan-1-ols binary systems. J. Chem. Eng. Data 47, 796–800 (2002)
Zarei, H.A., Mirhidari, N., Zangeneh, Z.: Densities, excess molar volumes, viscosity, and refractive indices of binary and ternary liquid mixtures of methanol (1) + ethanol (2) + 1,2-propanediol (3) at p=81.5 kPa. J. Chem. Eng. Data 54, 847–854 (2009)
Yang, C., Lai, H., Ma, P.: Densities and viscosities of diethyl carbonate + toluene, + methanol, and + 2-propanol from (293.15 to 363.15) K. J. Chem. Eng. Data 51, 584–589 (2006)
Changsheng, Y., Yifu, H., Peisheng, M.: Volumetric properties and viscosities of binary mixtures of N,N-dimethylformamide with methanol and ethanol in the temperature range (293.15 to 333.15) K. J. Chem. Eng. Data 53, 293–297 (2008)
Jian, H.Y., Yan, L.D., Zhong, X.W., Qi, Y.C.: Densities and viscosities of binary mixtures of cyclopropanecarboxylic acid with methanol, ethanol, propan-1-ol, and butan-1-ol at different temperatures. J. Chem. Eng. Data 54, 1147–1152 (2009)
Vercher, E., Llopis, F.J., Gonzalez-Alfaro, V., Martinez-Andreu, A.: Refractive indices and deviations in refractive indices for binary mixtures of 1-ethyl-3-methylimidazolium trifluoromethanesulfonate with methanol, ethanol, 1-propanol, and 2-propanol at several temperatures. J. Chem. Eng. Data 55, 1430–1433 (2010)
Orge, B., Rodriguez, A., Canosa, J.M., Marino, G., Iglesias, M., Tojo, J.: Variation of densities, refractive indices, and speeds of sound with temperature of methanol or ethanol with hexane, heptane, and octane. J. Chem. Eng. Data 44, 1041–1047 (1999)
Atomic weights of the elements 1995 (IUPAC technical report). Pure Appl. Chem. 68, 2339–2359 (1996)
Riddick, J.A., Bunger, W.B., Sakano, T.K.: Organic solvents. In: Weissberger, A. (ed.) Techniques of Chemistry, vol. II. Wiley, New York (1986)
Marsh, K.N.: Recommended Reference Materials for the Realization of Physical Properties. Blackwell Scientific, Oxford (1987)
Tamura, K., Ohomuro, K., Murakami, S.: Speeds of sound, isentropic and isothermal compressibilities, and isochoric heat-capacities of (xc-C6H12 + (1−x)C6H6), (xCCl4 + (1−x)C6H6), and (xC7H16 + (1−x)C6H6) at 298.15 K. J. Chem. Thermodyn. 15, 859–868 (1983)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 84–88 (1948)
Baragi, J.G., Aralaguppi, M.I., Kariduraganavar, M.Y., Kulkarni, S.S., Kitter, A.S., Aminabhavi, T.M.: Excess properties of the binary mixtures of methyl cyclohexane + alkanes (C6 to C12) at T=298.15 K to T=308.15 K. J. Chem. Thermodyn. 38, 75–83 (2006)
Garcia, B., Alcalde, R., Aparicio, S., Leal, J.M.: Volumetric properties, viscosities and refractive indices of binary mixed solvents containing methyl benzoate. Phys. Chem. Chem. Phys. 4, 5833–5840 (2002)
Gao, H., Qi, F., Wang, H.: Densities and volumetric properties of binary mixtures of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate with benzaldehyde at T=(298.15 to 313.15) K. J. Chem. Thermodyn. 41, 888–892 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Z., Gao, H., Wang, H. et al. Thermodynamic Properties of Binary Mixtures of the Amino Acid Ionic Liquids [Bmim][Glu] or [Bmim][Gly] with Methanol at T=298.15 to 313.15 K. J Solution Chem 41, 173–186 (2012). https://doi.org/10.1007/s10953-011-9788-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-011-9788-x