Abstract
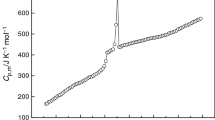
The molar heat capacities of an aqueous Li2B4O7 solution were measured with a precision automated adiabatic calorimeter in the temperature range from 80 to 356 K at a concentration of 0.3492 mol⋅kg−1. The occurrence of a phase transition was determined based on the changes in the curve of the heat capacity with temperature. A phase transition was observed at 271.72 K corresponding to the solid-liquid phase transition; the enthalpy and entropy of the phase transition were evaluated to be Δ H m = 4.110 kJ⋅mol−1 and Δ S m = 15.13 J⋅K−1⋅mol−1, respectively. Using polynomial equations and thermodynamic relationship, the thermodynamic functions [H T −H 298.15] and [S T −S 298.15] of the aqueous Li2B4O7 solution relative to 298.15 K were calculated in temperature range 80 to 355 K at intervals of 5 K. Values of the relative apparent molar heat capacities of the aqueous Li2B4O7 solution, C p,φ, were calculated at every 5 K in temperature range from 80 to 355 K from the experimental heat capacities of the solution and the heat capacities of pure water.
Similar content being viewed by others
References
Li, J., Song, P.S., Sun, B.: Synthesis and properties of dimagnesium hexaborate heptadecahydrate. Thermochim. Acta 233, 211–218 (1994)
Li, J., Li, B., Gao, S.Y.: Thermochemistry of hydrated potassium and sodium borates. J. Chem. Thermodyn. 30, 425–430 (1998)
Li, J., Gao, S.Y., Xia, S.P., Li, B., Hu, R.Z.: Thermochemistry of hydrated calcium borates. J. Chem. Thermodyn. 29, 1071–1075 (1997)
Li, J., Li, B., Gao, S.Y.: Thermochemistry of hydrated lithium borates. J. Chem. Thermodyn. 30, 681–688 (1998)
Zhang, A.Y., Yao, Y., Yang, J.M., Song, P.S.: Isopiestic determination of the osmotic coefficients and Pitzer model representation for Li2B4O7(aq) at T = 298.15 K. J. Chem. Thermodyn. 37, 101–109 (2005)
Tian, H.B., Yao, Y., Song, P.S.: Studies of activity coefficients of LiCl and association equilibrium in LiCl-Li2B4O7-H2O system at 298.15 K Chem. Res. Appl. (CA: Huaxue Yanjiu Yu Yingyong (in Chinese)) 12, 403–408 (2000).
Yang, J.M., Yao, Y., Zhang, A.Y., Song, P.S.: Isopiestic studies on thermodynamic properties for LiCl−Li2B4O7−H2O system at 298.15 K. J. Salt Lake Res. (in Chinese) 3, 31–38 (2004)
Zhang, A.Y., Yao, Y., Yang, J.M. Song, P.S.: Isopiestic studies of thermodynamic properties and representation with ion-interaction model for Li2B4O7-MgCl2(B)-H2O system at 298.15,K. Acta Chim. Sinica (CA: Huaxue Xuebao (in Chinese)) 62, 1089–1094 (2004)
Nan, Z.D., Tan, Z.C.: Measurements of the heat capacity of an azeotropic mixture of water, ethanol and toluene from 79 to 320 K. Fluid Phase Equilib. 226, 65–70 (2004)
Tan, Z.C., Zhou, L.X., Chen, S.X., Yin, A.X., Sun, Y., Ye, J.C., Wang, X.K.: An adiabatic calorimeter between heat capacity measurements from 80 to 400 K- heat capacities of α-alumina and n-heptane. Scientia Sinica (Series B) 26, 1014–1026 (1983)
Tan, Z.C., Sun, G.Y., Sun, Y., Yin, A.X., Wang, W.B., Ye, J.C., Zhou, L.X.: An adiabatic low-temperature calorimeter for heat capacity measurement of small samples. J. Thermal. Anal. 45, 59–67 (1995)
Tan, Z.C., Sun, L.X., Meng, S.H., Li, L., Xu, F.P.Y., Liu, B.P., Zhang, J.B.: Heat capacities and thermodynamic functions of p-chlorobenzoic acid. J. Chem. Thermodyn. 34, 1417–1429 (2002)
Nan, Z.D., Tan, Z.C.: Low-temperature heat capacity and derived thermodynamic functions of cyclohexane. J. Therm. Anal. Cal. 76, 955–963 (2004)
Tan, Z.C., Xue, B., Lu, S.W., Meng, S.H., Yuan, X.H., Song, Y.J.: Heat capacities and thermodynamic properties of fenpropathrin (C22H23O3N). J. Therm. Anal. Cal. 63, 297–308 (2001)
Spedding, F.H., Jones, K.C.: Heat capacities of aqueous rare earth chloride solutions at 25°. J. Phys. Chem. 70, 2450–2455 (1966)
Brown, B.R., Origlia-Luster, M.L., Niederhauser, T.L., Woolley, E.M.: Apparent molar volumes and heat capacities of aqueous lithium chloride, rubidium chloride, and cesium chloride at temperatures from (278.15 to 393.15) K at the pressure 0.35 MPa. J. Chem. Thermodyn. 36, 331–339 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, ZH., Yin, GY., Tan, ZC. et al. Heat Capacities and Thermodynamic Properties of a H2O + Li2B4O7 Solution in the Temperature Range from 80 to 356 K. J Solution Chem 35, 1347–1355 (2006). https://doi.org/10.1007/s10953-006-9065-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-006-9065-6