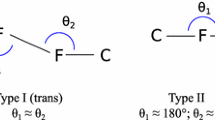
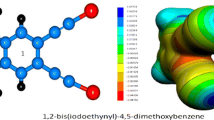
Abstract
Systematization of the available literature data on C-F...π, F...H, and F...F interactions, namely, statistical studies of the geometry of the corresponding contacts were carried out using the Cambridge Structural Database (CSD) and theoretical quantum-chemical estimations of their energies. The most typical supramolecular motifs (finite or infinite) involving the F atom were revealed based on recent X-ray studies of a few dozens of fluoroarenes carried out at the Novosibirsk Institute of Organic Chemistry. Our recent data were summarized. To assess the role of the above interactions, we used topological analysis of electron density distribution in terms of Bader’s QTAIM theory. Our DFT/PBE/3z quantum-chemical calculations of the interaction energies of molecular pairs in diazafluorene crystals formed by C-F...π, C-F...H, and F...F nonvalent short contacts are presented.
Similar content being viewed by others
References
G. R. Desiraju, Chem. Commun., 1475 (1997).
W. D. S. Motherwell, H. L. Ammon, J. D. Dunitz, et. al., Acta Crystallogr. B, 58, 647 (2002).
J. D. Dunitz and A. Gavezzotti, Cryst. Growth Des., 5, 2180 (2005).
A. Gavezzotti, J. Am. Chem. Soc., 113, 4622 (1991).
J. Marten, W. Seicher, et. al., Cryst. Eng. Commun., 10, 541 (2008).
F. Babudri, G. M. Farinola, F. Naso, and R. Ragni, Chem. Commun., 1003 (2007).
F. Leroux, P. Jeschke, and M. Schlosser, Chem. Rev., 105, 827 (2005).
P. Kirsch and M. Bremer, Angew. Chem., 112, 4384 (2000).
P. Kirsch and M. Bremer, Angew. Chem., Int. Ed., 39, 4216 (2000).
K. Kanie, K. Mizuno, M. Kuroboshi, et. al., Bull. Chem. Soc. Jpn., 72, 2523 (1999).
S. McLean, A. Ganong, A. Seymour, et. al., Pharm. Exp. Ther., 227, 900; PubMed ID 8627572 (1996).
K. Gehmann and R. Nyfeler, Chem. Abstr., 114. 223403 (1991).
K. Reichenbacher, I. Heike, H. I. Suss, and J. Jurg Hulliger, Chem. Soc. Rev., 34, 22 (2005).
E. Lork, R. Mews, M. M. Shakirov, et al., Eur. J. Inorg. Chem., 2123 (2001).
J. D. Dunitz, A. Gavezzotti, and W. B. Schweizer, Helv. Chim. Acta, 86, 4073 (2003).
F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 58, 380 (2002).
E. D’Oria and J. J. Novoa, Cryst. Eng. Commun., 10, 423 (2008).
R. S. Rowland and R. Taylor, J. Phys. Chem., 100, 7384 (1996).
V. R. Thalladi, H.-Ch. Weiss, D. Bläser, et. al., J. Am. Chem. Soc., 120, 8702 (1998).
G. R. Desiraju, Acc. Chem. Res., 35, 565 (2002).
L. C. Pauling, The Nature of the Chemical Bond, Cornell University Press, Ithaca (1960), p. 449.
I. Hyla-Kryspin, G. Haufe, and S. Grimme, Chem. Eur. J., 10, 3411 (2004).
E. Kryachko and S. Scheiner, J. Phys. Chem. A, 108, 2527 (2004).
W. Caminati, S. Melandri, P. Moreschini, and P. G. Favero, Angew. Chem., Int. Ed., 38, 2924 (1999).
J. Parsch and J. W. Engels, J. Am. Chem. Soc., 124, 5664 (2002).
J. A. K. Howard, V. J. Hoy, D. O’Hagan, and G. T. Smith, Tetrahedron., 38, 12613 (1996).
J. D. Dunitz and W. B. Schweizer, Chem. Eur. J., 12, 6804 (2006).
I. Yu. Bagryanskaya, Yu. V. Gatilov, A. M. Maksimov, et. al., J. Fluor. Chem., 126, 1281 (2005).
I. Yu. Bagryanskaya, M. A. Grishina, L. Yu. Safina, et al., J. Struct. Chem., 49, No. 5, 901–908 (2008).
N. Hayashi, T. Mori, and K. Matsumoto, Chem. Commun., 1905 (1998).
J. N. Williams, Acc. Chem. Res., 26, 593 (1993).
M. D. Prasana and T. N. Row Guru, Cryst. Eng., 3, 135 (2000).
V. M. Karpov, V. E. PLatonov, I. P. Chuikov, et al., Zh. Org. Khim., 40, No. 3, 448 (2004).
S. Lorenzo, G. R. Lewis, and I. Dance, New J. Chem., 24, 295 (2000).
R. F. W. Bader, Atoms in Molecules: Quantum Theory, Clarendon Press, Oxford (1990).
R. F. W. Bader, Atoms in Molecules: Quantum Theory, Clarendon Press, Oxford (1990).
R. F. W. Bader, Monat. Chem., 136, 819 (2005).
N. Ramasubbu, R. Parthasarathy, and P. Murray-Rust, J. Am. Chem. Soc., 108, 4308 (1986).
A. J. Bondi, Phys. Chem., 68, 441 (1964).
E. V. Bartashevich, M. R. Abdrakhmanova, V. A. Potemkin, and I. Yu. Bagryanskaya, J. Struct. Chem., 47, No. 1, 114–119 (2006).
O. V. Grineva and P. M. Zorkii, Zh. Fiz. Khim., 74, No. 11, 1937 (2000).
A. V. Zibarev, Yu. V. Gatilov, and A. O. Miller, Polyhedron, 11, No. 9, 1137 (1992).
I. Yu. Bagryanskaya, Yu. V. Gatilov, A. Yu. Makarov, et al., Heteroatom Chem., 10, No. 2, 113 (1999).
A. Yu. Makarov, I. Yu. Bagryanskaya, F. Van. Blockhuys, et al., Eur. J. Inorg. Chem., No. 1, 77 (2003).
A. Yu. Makarov, K. Tersago, K. Nivesanond, et al., Inorg. Chem., 45, 2221 (2006).
C. Knapp, E. Lork, R. Mews, and A. V, Zibarev, Eur. J. Inorg. Chem., 2446 (2004).
P. West, J. R. S. Mecozzi, and D. A. Dougherty, J. Phys. Org. Chem., 10, 3479 (1997).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2009 by T. V. Rybalova and I. Yu. Bagryanskaya
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 50, No. 4, pp. 767–779, July–August, 2009.
Rights and permissions
About this article
Cite this article
Rybalova, T.V., Bagryanskaya, I.Y. C-F...π, F...H, and F...F intermolecular interactions and F-aggregation: Role in crystal engineering of fluoroorganic compounds. J Struct Chem 50, 741–753 (2009). https://doi.org/10.1007/s10947-009-0113-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10947-009-0113-0