Abstract
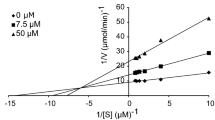
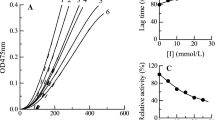
Tyrosinase inhibition studies are needed due to the agricultural and medicinal applications. For probing effective inhibitors of tyrosinase, a combination of computational prediction and enzymatic assay via kinetics were important. We predicted the 3D structure of tyrosinase from Agaricus bisporus, used a docking algorithm to simulate binding between tyrosinase and terephthalic acid (TPA) and studied the reversible inhibition of tyrosinase by TPA. Simulation was successful (binding energies for Autodock4 = −1.54 and Fred2.0 = −3.19 kcal/mol), suggesting that TPA interacts with histidine residues that are known to bind with copper ions at the active site. TPA inhibited tyrosinase in a mixed-type manner with a K i = 11.01 ± 2.12 mM. Measurements of intrinsic and ANS-binding fluorescences showed that TPA induced no changes in tertiary structure. The present study suggested that the strategy of predicting tyrosinase inhibition based on hydroxyl groups and orientation may prove useful for screening of potential tyrosinase inhibitors.









Similar content being viewed by others
Abbreviations
- DOPA:
-
3,4-Dihydroxyphenylalanine
- TPA:
-
Terephthalic acid
- ANS:
-
1-Anilinonaphthalene-8-sulfonate
References
Arnold K, Bordoli L, Kopp J, Schwede T (2006) Bioinformatics 22:195–201
Cui L, Dai G, Xu L, Wang S, Song L, Zhao R, Xiao H, Zhou J, Wang X (2004) Toxicology 201:59–66
Dai G, Cui L, Song L, Cheng J, Zhong Y, Zhao R, Wang X (2005) Food Chem Toxicol 43:217–224
Dai GD, Cui LB, Song L, Zhao RZ, Chen JF, Wang YB, Chang HC, Wang XR (2006) Biomed Environ Sci 19:8–14
Decker H, Tuczek F (2000) Trends Biochem Sci 25:392–397
Gheibi N, Saboury AA, Mansuri-Torshizi H, Haghbeen K, Moosavi-Movahedi AA (2005) J Enzyme Inhib Med Chem 20:393–399
Gou L, Lü ZR, Park D, Oh SH, Shi L, Park SJ, Bhak J, Park YD, Ren ZL, Zou F (2008) J Biomol Struct Dyn 26:395–402
Guerrero A, Rosell G (2005) Curr Med Chem 12:461–469
Han HY, Lee JR, Xu WA, Hahn MJ, Yang JM, Park YD (2007) J Biomol Struct Dyn 25:165–171
Huey R, Morris GM, Olson AJ, Goodsell DS (2007) J Comput Chem 28:1145–1152
Jimbow K, Park JS, Kato F, Hirosaki K, Toyofuku K, Hua C, Yamashita T (2000) Pigment Cell Res 13:222–229
Kanade SR, Suhas VL, Chandra N, Gowda LR (2007) FEBS J 274:4177–4187
Kanost MR, Jiang H, Yu XQ (2004) Immunol Rev 198:97–105
Khatib S, Nerya O, Musa R, Shmuel M, Tamir S, Vaya J (2005) Bioorg Med Chem 13:433–441
Kim D, Park J, Kim J, Han C, Yoon J, Kim N, Seo J, Lee C (2006) J Agric Food Chem 54:935–941
Kim YJ, Uyama H (2005) Cell Mol Life Sci 62:1707–1723
Lai SC, Chen CC, Hou RF (2002) J Med Entomol 39:266–274
Lape M, Elam C, Paula S (2010) Biophys Chem 150:88–97
Li Y, Wang Y, Jiang H, Deng J (2009) Proc Natl Acad Sci USA 106:17002–17006
Lü ZR, Shi L, Wang J, Park D, Bhak J, Yang JM, Park YD, Zhou HW, Zou F (2010) Appl Biochem Biotechnol 160:1896–1908
Matoba Y, Kumagai T, Yamamoto A, Yoshitsu H, Sugiyama M (2006) J Biol Chem 281:8981–8990
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) J Comput Chem 30:2785–2791
Olivares C, Solano F (2009) Pigment Cell Melanoma Res 22:750–760
Park KH, Lee JR, Hahn HS, Kim YH, Bae CD, Yang JM, Oh S, Bae YJ, Kim DE, Hahn MJ (2006) Chem Pharm Bull 54:1266–1270
Park YD, Kim SY, Lyou YJ, Lee JY, Yang JM (2005) Biochimie 87:931–937
Park YD, Kim SY, Lyou YJ, Lee DY, Yang JM (2006) Biochem Cell Biol 84:112–116
Park YD, Lyou YJ, Hahn HS, Hahn MJ, Yang JM (2006) J Biomol Struct Dyn 24:131–138
Rescigno A, Sollai F, Pisu B, Rinaldi A, Sanjust E (2002) J Enzyme Inhib Med Chem 17:207–218
Sendovski M, Kanteev M, Ben-Yosef VS, Adir N, Fishman A (2011) J Mol Biol 405:227–237
Shiino M, Watanabe Y, Umezawa K (2001) Bioorg Med Chem 9:1233–1240
Shiino M, Watanabe Y, Umezawa K (2003) Bioorg Chem 31:129–135
Xie MX, Xu XY, Wang YD (2005) Biochim Biophys Acta 1724:215–224
Yamazaki Y, Kawano Y, Yamanaka A, Maruyama S (2009) Bioorg Med Chem Lett 19:4178–4182
Yan Q, Cao R, Yi W, Yu L, Chen Z, Ma L, Song H (2009) Bioorg Med Chem Lett 19:4055–4058
Yokota T, Nishio H, Kubota Y, Mizoguchi M (1998) Pigment Cell Res 11:355–361
Yoon J, Fujii S, Solomon EI (2009) Proc Natl Acad Sci USA 106:6585–6590
Zhu YJ, Qiu L, Zhou JJ, Guo HY, Hu YH, Li ZC, Wang Q, Chen QX, Liu B (2010) J Enzyme Inhib Med Chem 25:798–803
Acknowledgments
This research was supported by Consultation Program funded by the Science and Technology Department of Zhejiang Province (2008C0200-2). Dr. Jun-Mo Yang was supported by the grants of the Korea Health 21 R&D Project (Ministry of Health, Welfare and Family Affairs, Republic of Korea, 01-PJ3-PG6-01GN12-0001 and A030003). Dr. Yong-Doo Park was supported by a grant from the project of Zhejiang Provincial Natural Science Foundation of China (Grant No. Y2091212).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yin, SJ., Si, YX., Chen, YF. et al. Mixed-Type Inhibition of Tyrosinase from Agaricus bisporus by Terephthalic Acid: Computational Simulations and Kinetics. Protein J 30, 273–280 (2011). https://doi.org/10.1007/s10930-011-9329-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-011-9329-x