Abstract
To elucidate the role of leucine 134 of Bacillus licheniformis nucleotide exchange factor (BlGrpE), site-saturation mutagenesis was employed to generate all possible replacements for this residue. Wild-type and mutant proteins were purified by nickel-chelated chromatography and had a molecular mass of approximately 34.5 kDa. As compared with wild-type BlGrpE, the nucleotide exchange factor (NEF) activity of L134H, L134K, L134R, L134D, L134E, L134N, L134Q, L134S, L134G and L134P was reduced by more than 96%. In vitro binding assay revealed that wild-type BlGrpE and the functional variants mainly interacted with the monomer of BlDnaK, but no such interaction was observed for the remaining mutant proteins. BlGrpE and 9 mutant proteins synergistically stimulated the ATPase activity of B. licheniformis DnaK (BlDnaK), whereas the NEF-defective variants had no synergistic stimulation. Comparative analysis of the far-UV CD spectra showed that the α-helical content of the inactive mutant BlGrpEs was reduced significantly with respect to wild-type protein. Moreover, the inactive mutant proteins also exhibited a more sensitivity towards the temperature-induced denaturation. Taken together, these results indicate that Leu134 might play a structural role for the proper function of BlGrpE.
Similar content being viewed by others
1 Introduction
Molecular chaperones are involved in various cellular functions including protein folding, refolding/degradation of nonnative proteins, prevention of protein aggregation under stress condition, and regulation of protein activities [18]. Heat shock protein 70 (Hsp70) is a ubiquitous molecular chaperone family widely distributed in the three domains of life. Hsp70 is an ATP-dependent molecular chaperone that exhibits weak intrinsic ATPase activity and the protein is usually present in an ATP-bound state [27]. Although Hsp70 has low affinity together with fast exchange rates for substrates in the ATP-bound state, ADP-bound Hsp70 undergoes an internally conformational change that results in comparatively high affinity coupled with slow exchange rates towards substrates [8]. This structural alteration of Hsp70 is tied with ATP hydrolysis and ADP/ATP exchange, which is closely related to the actions of the co-chaperones J-protein and nucleotide exchange factor (NEF) [8]. Both cooperative proteins are essential for the efficient progression of the molecular chaperone cycle.
In prokaryotes, Escherichia coli DnaK (EcDnaK) is a well-known Hsp70 counterpart whose ATPase activity is mediated by an Hsp40 protein, DnaJ, in synergy with substrate binding [9]. It has been shown that ADP dissociation from EcDnaK is accelerated 5,000-fold by an NEF homologue, GrpE [36] and such action leads to the efficient ATP rebinding to the molecular chaperone and substrate release [26]. E. coli GrpE (EcGrpE) consists of a homodimeric structure with a very unusual and unique feature of two long α-helices at the N-terminal end paired together in a non-coiled parallel arrangement [17]. This tail region of the structure has been shown to act as a thermosensor in the temperature-regulated action of the DnaK/DnaJ/GrpE heat shock system [14, 40]. There is also a four-helix bundle formed at the dimer interface with each monomer contributing two α-helices and it has been shown that the bundle serves as a scaffold for the association of the long helices [12, 30]. The crystal structure has demonstrated a direct interaction between EcGrpE and the nucleotide-binding domain (NBD) of EcDnaK, suggesting a forced opening of the nucleotide-binding cleft of EcDnaK by insertion of the β-sheet domain of EcGrpE [17]. Very recently, the structure of Thermus thermophilus GrpE was determined at 3.23-Å resolution and has shed still more light on the topology of this protein group [35].
Earlier, a recombinant B. licheniformis GrpE (BlGrpE) was expressed in E. coli cells [23], and a double mutant BlGrpE (BlGrpE-L52P/L134H) with no co-chaperone function was also characterized [22]. In order to do a further investigation at leucine 134 of BlGrpE, we performed saturation mutagenesis on this position. Surprisingly, it was found that some replacements were detrimental to the structural integrity of BlGrpE. This clearly brings into the suggestion that Leu134 is necessary for BlGrpE to function as a co-chaperone. The saturation mutagenesis also indentified the functionally competent replacements that had a comparable co-chaperone activity with respect to wild-type BlGrpE.
2 Materials and Methods
2.1 Materials, Bacterial Strains, and Growth Conditions
Luria-Bertani (LB) media for bacterial culture were acquired from Difco Laboratories (Detroit, MI, USA). A QuikChange II site-directed mutagenesis kit for mutagenic PCR amplification was purchased from Stratagene (La Jolla, CA, USA). Protein assay reagents were obtained from Bio-Rad Laboratories (Hercules, CA, USA). Ni2+-nitrilotriacetate (Ni2+-NTA) resin was acquired from Qiagen Inc. (Valencia, CA, USA). Unless otherwise noted, all other chemicals were commercial products of analytical grade or molecular biological grade.
Escherichia coli NovaBlue (Novagen Inc., Madison, WI, USA) was used in the preparation of plasmids and E. coli XL-1 Blue (Stratagene) was used for site-directed mutagenesis. T5 RNA-polymerase-mediated gene expression was performed in E. coli M15 (pRep4) (Qiagen). E. coli strains were grown aerobically in LB medium at either 20 or 37 °C. As required, ampicillin and kanamycin were supplemented to a final concentration of 100 and 25 μg/ml, respectively.
2.2 Saturation Mutagenesis
The L134X-mutated grpE genes from B. licheniformis were constructed on the expression plasmid pQE-BlGrpE [23] by oligonucleotide-directed mutagenesis using the following complementary primers: 5′-ATTTTGGAGGCTNNNAAAAATGAAGGAGTT-3′ and 5′-AACTCCTTCATTTTTNNNAGCCTCCAAAAT-3′. Underlined sequences, which encode codon at site 134, were randomized for saturation mutagenesis. Mutant DNAs were generated with a thermocycling program of 2 min at 95 °C and 16 cycles of 30 s at 95 °C, 60 s at 55 °C, and 12 min at 68 °C on an Applied Biosystems thermal cycler. The amplified products were digested with 10 units of DpnI at 37 °C for 1 h, prior to their use for transformation into E. coli XL-1 blue cells. Mutations were confirmed by DNA sequencing, which was carried out with dye terminator sequencing kit and an automatic DNA sequencer (Applied Biosystems, Foster City, CA, USA).
2.3 Expression and Purification of Wild-type and Mutant Proteins
To express wild-type and mutant BlGrpEs, plasmid constructions were transformed into the E. coli M15 (pRep4). Transformed cells were grown at 37 °C, with shaking in LB broth containing 100 μg ampicillin/ml and 25 μg kanamycin/ml, until a cell density (optical density at 600 nm) of 0.6–0.8 was reached. Flasks containing the cultures were supplemented with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 1 mM. Cells were then cultured at 20 °C for 14 h with vigorous shaking. Cells were collected and pellets were resuspended in a buffer containing 50 mM Tris–HCl, 300 mM NaCl, and 10 mM imidazole buffer at pH 7.0. Cells were lysed by sonication (30-s bursts and pulses for 5 min), and cell debris was removed by centrifugation at 12,000g. Recombinant proteins were purified by affinity chromatography using a Ni2+-NTA agarose column (Qiagen) under native conditions.
B. licheniformis DnaK (BlDnaK) and DnaJ (BlDnaJ) were expressed and purified as described previously [23]. To remove nucleotides bound to BlDnaK, the purified protein (0.24 mg/ml) was diluted with 50 mM Tris-HCl buffer (pH 7.0) to 50 ml, and ten units of alkaline phosphatase (Promega Corp., Madison, WI, USA) were added and allowed to react at room temperature for one hour. Then, the solution was concentrated to 5 ml using an Amicon stirred cell equipped with YM-10 membrane (Millipore Corp., Billerica, MA, USA) and applied to a Superdex 200-gel filtration column (Pharmacia) for removal of alkaline phosphatase. After analysis of the steady-state turnover rate of ATP by a coupled colorometric assay [1], protein fractions with a turnover number of at most 1.5 × 10−3 s−1 were pooled and stored at −70 °C until use.
2.4 Electrophoresis and Determination of Protein Concentration
Polyacrylamide gel electrophoresis (PAGE) was performed on 7.5% native-gel and 12% SDS-gel using a mini-slab gel system (Mini Protean III; Bio-Rad). Following electrophoresis, the proteins were stained with Coomassie Brilliant Blue-250 and destained with a methanol (30%) and acetic acid (10%) solution. The protein standards used for estimation of molecular masses were rabbit phosphorylase b (97.4 kDa), bovine serum albumin (66.2 kDa), chicken egg albumin (45.0 kDa), bovine carbonic anhydrase (29.0 kDa), and trypsin inhibitor (20.1 kDa).
Protein concentrations were determined using the Bio-Rad protein assay based on the Bradford dye-binding procedure [6] with bovine serum albumin as the standard.
2.5 Nucleotide Release Measurements
Nucleotide release experiments of the fluorescent nucleotide analog MABA-ADP (MoBiTec GmbH, Goettingen, Germany) was performed with an SX20 stopped-flow spectrometer (Applied Photophysics, Surrey, UK) as described elsewhere [7]. To obtain spontaneous nucleotide release rates, 2.5 μM BlDnaK-MABA-ADP was mixed at 30 °C with an equal volume of 250 μM ADP (both in 50 mM Tris–HCl buffer, pH 7.0, containing 50 mM KCl and 5 mM MgCl2). The nucleotide exchange activity of wild-type and mutant BlGrpEs was assayed by adding the protein samples to the ADP solution and subsequently mixed with BlDnaK-MABA-ADP complex. Release of labeled nucleotides was monitored by the decrease in fluorescence of the unbound MABA-ADP (excitation at 360 nm and cutoff filter at 420 nm).
2.6 ATPase Activity Assay
Steady-state ATPase activity assays were performed according to the malachite green method [21] with slight modifications. The reaction mixture contained 10 mM Hepes buffer (pH 7.0), 100 mM KCl, 2.5 mM MgCl2, 1 mM ATP and 25 μM purified BlDnaK. The release of free phosphate was recorded by monitoring the color changes at 660 nm. The assays were corrected for spontaneous ATP degradation. ATPase activity is defined as pmol ATP pmol BlDnaK−1 min−1 under the assay conditions.
Mutational effects on the ATPase activity of BlDnaK (25 μM) were investigated in the presence of B. licheniformis DnaJ (BlDnaJ) and NR-peptide (NRLLLTG). The reaction mixture contained the above-mentioned buffer system, 100 μM each of the recombinant BlGrpEs, 25 μM BlDnaK, 50 μM BlDnaJ, and 50 μM NR-peptide. The ATPase activity was determined under the standard assay conditions.
2.7 Circular Dichroism (CD) Studies
Far-UV CD experiments were performed on a JASCO J-815 spectropolarimeter equipped with a temperature-controlling system. A stock of BlGrpE or its derivatives was diluted in 10 mM Hepes buffer (pH 7.0) to a final concentration of 30 μM. Spectral analysis was taken over the wavelength range from 190 to 250 nm in cuvettes with a 2-mm path length at 0.2-nm intervals with 4-s integration time and a bandwidth of 2.0 nm. All of the measurements were performed under nitrogen flow. Each scanning was repeated four times and an average was obtained. Data were corrected for the effect of buffer, and the results were expressed as mean residue ellipticity [θ] in the unit of degrees cm2 dmol−1, which is defined as [θ] = M × θ/(100 × c × l), where M is the average molecular mass of amino acids, θ is the observed ellipticity in degrees, c is the concentration in residue moles per liter, and l is the length of the light path in centimeters. The secondary structural contents were estimated by the online DICHROWEB server (http://public-1.cryst.bbk.ac.uk/cdweb/htm1/, accessed on March 4, 2010) [25, 45] using the implemented CDSSTR program.
The unfolding transition curves for the recombinant proteins were obtained by measuring the ellipticity at 222 nm in a 1-mm cell at a protein concentration of 35 μM. The temperature was increased with a heating rate of 1 °C/min from 20 to 90 °C. For refolding experiments, the temperature was decreased by 1 °C/min and measurements were taken once every min. Thermal denaturation curves were fitted to a modified form of the van’t Hoff equation, which simultaneously fits the native and denatured baselines and the transition region to obtain the T m and ΔH values for denaturation [16]:
where
Here, m n and m d, and b n and b d are the slopes and intercepts of the native- and denatured-state baselines respectively and T is the temperature.
3 Results and Discussion
3.1 Sequence Comparison
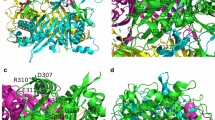
Intensive biochemical and structural studies of GrpE proteins provide a solid foundation for the investigation into the residues or regions crucial for the co-chaperone activity [33, 38, 42, 46–48]. The lack of structural data for BlGrpE has invited speculations about possible similarities to other GrpE proteins. In this study, we aligned the amino acid sequence of BlGrpE with that of EcGrpE, whose three-dimensional structure has been determined [17]. By performing the program CLUSTLAW from ExPASy Proteomics server (http://tw.expasy.org), we observed that the BlGrpE sequence shares 29% identity with the primary sequence of EcGrpE. The alignment also indicated these two GrpE proteins have common structural features, especially the domain organization, with the N-terminal long α-helix, four-helix bundle, and C-terminal β-sheet domains arranged in order (Fig. 1). Therefore, it can be expected that the fundamental mechanism of the NEF reaction for DnaK by GrpE is sufficiently conserved between B. licheniformis and E. coli DnaK systems. As shown in Fig. 1, Leu134 is located at the boundary of the α3-helix of BlGrpE. However, the role of this residue to the co-chaperone activity of BlGrpE remains unclear. A further investigation will contribute a more comprehensive understanding to its importance in BlGrpE function.
Amino acid sequence alignment of BlGrpE and EcGrpE proteins. Based on the crystal structure of EcGrpE, the secondary-structure elements of BlGrpE are illustrated below the sequences. The identical residues are shaded and EcGrpE residues interacting with EcDnaK in the crystal structure of their complex are represented with closed circles [7]
3.2 Site-saturation Mutagenesis and Purification of the Recombinant Proteins
Site-saturation mutagenesis provides a platform for the rapid diversification of protein traits [31]. This approach makes all possible mutations at one or pre-determined target positions and has been used successfully for in vitro directed evolution of various proteins [3, 20, 31, 37]. In this study, site-saturation mutagenesis at leucine 134 was employed to investigate in greater depth the contribution of this residue to the co-chaperone function of BlGrpE. A library of BlGrpE variants was created by overlap extension PCR using two degenerate synthetic oligonucleotides in which the target site (codon 134) was diversified with a randomized NNN codon. The library was subsequently screened by DNA sequencing for clones with the desired mutations. After the screening, pQE-BlGrpE and each of the mutated plasmids were transformed into E. coli M15 (pRep4) for IPTG-induced gene expression. The expression of wild-type and mutant BlGrpEs was assessed by analyzing protein profiles of the crude extracts. Both wild-type and mutant BlGrpEs were expressed as a predominant band with an estimated molecular mass of 34.5 kDa (data not shown), which is inconsistent with the expected molecular mass of approximately 23.8 kDa. These results indicate that the recombinant BlGrpEs displayed an abnormal mobility on SDS-PAGE. Interestingly, the anomalous electrophoretic behavior has also been observed in His6-tagged GrpE from E. coli [41]. Few acidic proteins, such as ribonuclease U2 [11], α-synuclein [34], Gir2 [2] and rhizopuspepsin [10], have high content of acidic amino acids (14-25%) and migrate slower than expected. BlGrpE also contains high proportion of acidic amino acids (23.4%), which may be responsible for its slower migration on SDS-PAGE. Wild-type and mutant proteins in the crude extracts were further purified to near homogeneity by Ni2+-NTA resin (Fig. 2). The purification procedure resulted in a final yield of approximately 5–11 mg protein per liter of cell culture.
3.3 Functional Analysis of Wild-type and Mutant BlGrpEs
To evaluate the mutational effects on the functionality of BlGrpE, wild-type and mutant proteins were tested for nucleotide exchange activity on BlDnaK using the stopped-flow assay. As shown in Table 1, the release rate of MABA-ADP was reduced more than 96% relative to wild-type BlGrpE when Leu134 of the protein was replaced with His, Lys, Arg, Asp, Glu, Asn, Gln, Ser, Gly, and Pro. Additionally, the dramatic loss of NEF activity was correlated with the incapable binding of the mutant proteins to BlDnaK (Table 1). These findings suggest that residue Leu134 of BlGrpE is essential for the functional and physical interactions with BlDnaK.
3.4 DnaK-binding Ability of Wild-type and Mutant BlGrpEs
In solution, GrpE is an elongated homodimer that consists of an N-terminal unstructured region of unknown function, a long α-helix that forms two coils in the dimer, a four-helix bundle with two helices from each monomer responsible for protein dimerization and a C-terminal compact β-sheet domain [17]. The nucleotide exchange activity is based on its interaction with DnaK that induces a 14o outward rotation of subdomain IIB, disrupting the nucleotide-binding site and decreasing the affinity for ADP. In the three dimensional structure of the EcDnaKNBD:EcGrpE complex, the dimer is asymmetric and the contacts are situated within the β-sheet and four-helix bundle subdomains of EcGrpE [17]. Partial proteolysis and substrate dissociation kinetics further suggest that the N-terminal half of EcGrpE interacts with the interdomain linker of EcDnaK, regulates the nucleotide exchange activity of the co-chaperone and is required to stabilize DnaK-substrate complexes in the ADP-bound conformation [33].
To evaluate the interactions between BlDnaK and the purified BlGrpEs, each (~15 μM) of the recombinant proteins in 10 mM Hepes buffer (pH 7.0) containing 100 mM KCl, 2.5 mM MgCl2, and 0.5 mM DTT was incubated with the molecular chaperone (7 μM) at 4 °C for 40 min. These samples were then subjected to gel electrophoresis under native conditions. Interestingly, the electrophoretic migrations for the monomeric form of wild-type and mutant BlGrpEs showed a significant difference (data not shown), especially L134R, L134D, L134E and L134 N, although they had a similar molecular mass. It should be noted that the monomer was the predominant form of BlDnak, with a small fraction of other oligomeric species. Oligomerization is a common feature of Hsp70 homologs including Hsc70 [4], EcDnaK [39], and Agrobacterium tumefaciens DnaK [5]. Non-denaturing PAGE analysis also revealed that wild-type BlGrpE and the functional variants were specifically bound to the monomer of BlDnaK (Table 1). However, non-specific bindings to BlDnak were observed in L134H, L134K, L134R, L134D, L134E, L134N, L134Q, L134S, L134G and L134P, implying that the relevant replacements result in the significant changes in the BlGrpE structure.
3.5 Co-chaperone Activity of the Purified BlGrpEs
It has been demonstrated that the chaperone function of DnaK is based on the substrate-binding and -release cycles, which are tightly regulated by nucleotides and co-chaperones DnaJ and GrpE [24, 28, 43]. In the ATPase cycle of DnaK, ATP-hydrolysis step and nucleotide-exchange step are rate-limiting processes in which DnaJ accelerates the hydrolysis rate of the DnaK-bound ATP and GrpE increases the release rate of bound ADP without affecting the rate of ATP hydrolysis. By their synergistic function, the apparent ATPase activity of DnaK is greatly stimulated in the presence of co-chaperones. In this regard, co-chaperone activity of GrpE should be evaluated to confirm whether the mutant proteins are active. The intrinsic specific ATPase activity for BlDnaK was determined to be 0.39 ± 0.05 pmol ATP pmol BlDnak−1 min−1. Upon the addition of wild-type BlGrpE, the specific activity was increased to 0.97 ± 0.07 pmol ATP pmol BlDnak−1 min−1. As compared with wild-type BlGrpE only, the simultaneous addition of BlDnaJ and BlGrpE synergistically stimulated the ATPase activity of BlDnaK by 8.7-fold (data not shown) and a further enhancement (12.5-fold) was observed by the incorporation of NR-peptide into the reaction mixture (Table 1). Similar to wild-type BlGrpE, the ATPase activity of BlDnaK was stimulated by more than 12-fold in the presence of some mutant BlGrpEs (including L134F, L134Y, L134C, L134I, L134M and L134V), BlDnaJ, and NR-peptide. However, the incorporation of L134H, L134K, L134R, L134D, L134E, L134N, L134Q, L134S, L134G, and L134P had no significant stimulation on the ATPase activity of BlDnaK. These results suggest that some replacements, especially the introduction of a polar group or a charge group into position 134, had impaired the functionality of BlGrpE.
3.6 Structural Analyses
To analyze how the secondary structure of BlGrpE was affected by the mutations, we measured far-UV CD properties of wild-type and mutant proteins. The CD spectrum of BlGrpE displays strong peaks of negative ellipticity at 208 and 222 nm indicative of substantial α-helical content (Fig. 3). This spectrometric characteristic strongly resembles those of GrpE counterparts from E. coli [12], T. thermophilus [15], Chlamydomonas reinhardtii chloroplast [46], and yeast mitochondria [32]. Most of the mutant BlGrpEs had a CD spectrum comparable to the wild-type protein, whereas the ellipticity was reduced greatly in L134D, L134E, L134H, L134N, L134P, L134Q, and L134R (Fig. 3). The spectra were further quantitatively analyzed by the DICHROWEB server. The normalized root-mean-square deviation values of the data fitting for wild-type and mutant BlGrpEs were all <0.1 units and thus showed excellent quality of the fit parameters [29]. The helical contents of BlGrpE, L134Y, L134W, L134F, L134C, L134V, L134M, and L134I were 50, 47, 47, 57, 64, 46, 56, and 54%, respectively, and the β-sheet contents were 13, 11, 13, 8, 9, 10, 11, and 20%, respectively. The calculated helical and β-sheet contents of BlGrpE were similar to the data derived from the crystal structure of GrpE counterpart from E. coli (PDB code: 1dkg). It is worth noting that the helical contents of inactive mutant BlGrpEs were apparently lower than that of wild-type BlGrpE (data not shown). These values confirm that the significant changes in the secondary structure of BlGrpE have been occurred as a consequence of L134R/K/H/E/D/T/S/Q/N/P mutations.
As noted by Grimshaw et al. [13], EcGrpE is the only component of the DnaK system that undergoes conformational changes in the physiological temperature range. Conformational changes of EcGrpE are fully reversible and it becomes transiently unable to interact with the NBD of EcDnaK at heat-shock temperatures, leading to stabilization of the DnaK-ADP-substrate complex. In this regard, the temperature-induced unfolding transitions of wild-type and mutant BlGrpEs followed by the loss of ellipticity at 222 nm with increasing temperature was investigated (Fig. 4). The transition for wild-type BlGrpE started at ~42 °C and had its midpoint at 58 °C. It has been reported that EcGrpE has two thermal transitions with the midpoint temperatures at 53 and 83 °C [10]. The first transition was proposed to attribute the unfolding of the paired α-helices and the second transition was assumed to involve in the unfolding of the four-helix bundle and dissociation of the dimer [12, 13]. However, only one fully reversible transition was observed in a GrpE homolog of yeast mitochondria and this transition was believed to be responsible for unfolding and dimer dissociation of the protein [32]. Consistent with the counterpart of yeast mitochondria, wild-type and mutant BlGrpEs showed only one transition and this transition was highly reversible (data not shown). The functional mutant BlGrpEs had their T m values comparable to the wild-type protein, whereas the midpoint temperatures for the inactive variants were dramatically reduced to less than 47 °C. These results clearly indicate that substitution of leucine 134 with polar or charged amino acids has a detrimental effect on the thermal stability of BlGrpE.
The thermodynamic parameters of the inactivation process were also analyzed. As shown in Table 2, the ΔH value for wild-type BlGrpE was calculated to be 91.7 ± 3.9 kcal/mol; however, this value was reduced to <52 kcal/mol in the inactive variants. Principally, the stability of the native state results from a balance between enthalpy and entropy. It has been demonstrated that the stabilization of proteins is mostly accompanied by a decrease in ΔS [44]. In our case, we should pay attention to the entropic increase of the unfolded state of the mutant BlGrpEs relative to that of the wild-type protein (Table 2). An increase in the entropy of the inactive variants implies that the relevant substitutions have a detrimental effect on the thermostability of BlGrpE. Proteins are only marginally stable since the free energy of stabilization (ΔG N→U) ranges within 7.1–15.4 kcal/mol and is therefore equivalent to only a few weak bonds [19]. Therefore, it is not surprising that their stability can be weakened through the lack of a few intramolecular interactions. As shown in Table 2, a significant reduction in ΔG N→U values of the inactive variants reflects that these mutant proteins are unstable in the native state.
4 Conclusion
In summary, the experimental data show how mutations of a single residue at the edge of α-helx 3 of BlGrpE can affect its functionality. Interestingly, the alternative choices for position 134 of BlGrpE could be phenylalanine, tryptophan, threonine, tyrosine, cysteine, isoleucine, methionine, and valine. The relevant replacements generate many variants with biochemical and biophysical characteristics comparable to wild-type BlGrpE. Therefore, these results might provide a clue for the evolutionary trajectory of this protein group.
Abbreviations
- Hsp70:
-
Heat shock protein 70
- NEF:
-
Nucleotide exchange factor
- EcDnaK:
-
Escherichia coli DnaK
- EcGrpE:
-
E. coli GrpE
- NBD:
-
Nucleotide-binding domain
- BlGrpE:
-
Bacillus licheniformis GrpE
- BlDnaK:
-
B. licheniformis DnaK
- Ni2+-NTA:
-
Ni2+-nitrilotriacetate
- IPTG:
-
Isopropyl-β-d-thiogalactopyranoside
- PAGE:
-
Polyacrylamide gel electrophoresis
- BlDnaJ:
-
B. licheniformis DnaJ
References
Adam H (1962) Adenosin 5’-diphosphat und Adenosin 5’-monophosphate. In: Bergmeyer HU (ed) Methoden der enzymatischen analyse. Verlag Chemie, Weinheim, pp 573–577
Alves VS, Pimenta DC, Sattlegger E, Castilho BA (2004) Biochem Biophys Res Commun 314:229–234
Armstrong Z, Reitinger S, Kantner T, Withers SG (2010) Chembiochem 11(4):533–538
Benaroudj N, Batelier G, Triniolles F, Ladjimi MM (1995) Biochemistry 34:15282–15290
Boshoff A, Stephens LL, Blatch GL (2008) Int J Biochem Cell Biol 40:804–812
Bradford MM (1976) Anal Biochem 72:248–254
Brehmer D, Rudiger S, Gassler CS, Klostermeier D, Packschies L, Reinstein J, Mayer MP, Bukau B (2001) Nat Struct Biol 8:427–432
Bukau B, Horwich AL (1998) Cell 92:351–366
Bukau B, Weissmam J, Horwich A (2006) Cell 125:443–451
Chen CC, Cho YC, Lai CC, Hsu WH (2009) J Agric Food Chem 57(15):6742–6747
Garcia-Ortega L, de Los Ríos V, Martinez-Ruiz A, Onaderra M, Lacadena J, Martinez del Pozo A, Gavilanes JG (2005) Electrophoresis 26:3407–3413
Gelinas AD, Langsetmo K, Toth J, Bethoney KA, Stafford WF, Harrison CJ (2002) J Mol Biol 323:131–142
Grimshaw JPA, Jelesarov I, Siegenthaler RK, Christen P (2001) J Biol Chem 276:6098–6104
Grimshaw JPA, Jelesarov I, Siegenthaler RK, Christen P (2003) J Biol Chem 278:19048–19053
Groemping Y, Klostermeier D, Herrmann C, Veit T, Seidel R, Reinstein J (2001) J Mol Biol 305:1173–1183
Eftink MR, Ramsay GD (1994) Methods Enzymol 240:615–645
Harrison CJ, Hoyer-Hartl M, Di Liberto M, Hartl F, Kuriyan J (1997) Science 276:431–435
Hartl FU, Hayer-Hartl M (2002) Science 295:1852–1858
Jaenicke R (1991) Eur J Biochem 202:715–728
Kotzia GA, Labrou NE (2009) FEBS J 276:1750–1761
Lanzett PA, Alvarez LJ, Reinach PS, Candia OA (1979) Anal Biochem 100:95–97
Liang WC, Lin MG, Chi MC, Chang HP, Lin LL (2009) Int J Biol Macromol 45:352–358
Liang WC, Lin MG, Chi MC, Hu HY, Lo HF, Chang HP, Lin LL (2009) Arch Microbiol 191:583–593
Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M (1991) Proc Natl Acad Sci USA 88:2874–2878
Lobley A, Whitmore L, Wallace BA (2002) Bioinformatics 18:211–212
Mally A, Witt SN (2001) Nat Struct Biol 8:254–257
Mayer MP, Rüdiger S, Bukau B (2000) Biol Chem 381:877–885
Mayer MP, Schröder H, Rüdiger S, Paal K, Laufen T, Bukau B (2000) Nat Struct Biol 7:586–593
Mao D, Wachter E, Wallace BA (1982) Biochemistry 21:4960–4968
Mehl AF, Heskett LD, Jain SS, Demeler B (2003) Protein Sci 12:1205–1215
Miyazaki K, Arnold FH (1999) J Mol Evol 49:716–720
Moro F, Muga A (2006) J Mol Biol 358:1367–1377
Moro F, Taneva SG, Velázquez-Campoy A, Muga A (2007) J Mol Biol 374:1054–1064
Moussa CE, Wersinger C, Rusnak M, Tomita Y, Sidhu A (2004) Neurosci Lett 371:239–243
Nakamura A, Takumi K, Miki K (2010) J Mol Biol 396:1000–1011
Packschies L, Theyssen H, Buchberger A, Bukau B, Goody RS, Reinstein J (1997) Biochemistry 36:3417–3422
Padhi SK, Bougioukou DJ, Stewart JD (2009) J Am Chem Soc 131(9):3271–3280
Popp SL, Reinstein J (2009) FEBS Lett 583:575–578
Schönfeld HJ, Schmidt D, Schröder H, Bukau B (1995) J Biol Chem 270:2183–2189
Siegenthaler RK, Christen P (2005) J Biol Chem 280:14395–14401
Sugimoto S, Higashi C, Yoshida H, Sonomoto K (2008) Protein Exp Purif 60:31–36
Sugimoto S, Saruwatari K, Higashi C, Sonomoto K (2008) Microbiology 154:1876–1885
Suh WC, Lu CZ, Gross CA (1999) J Biol Chem 274:30534–30539
Vieille C, Zeikus JG (2001) Trends Biotechnol 14:183–190
Whitmore L, Wallace BA (2004) Nucleic Acids Res 32:W668–W673
Willmund F, Mühlhaus T, Wojciechowska M, Schroda M (2007) J Biol Chem 282:11317–11328
Wu B, Wawrzynow A, Zylicz M, Georgopoulos C (1996) EMBO J 15:4806–4816
Zmijewski MA, Skórko-Glonek J, Tanfani F, Banecki B, Kotlarz A, Macario AJ, Lipińska B (2007) Acta Biochim Pol 54:245–252
Acknowledgments
This work was supported by National Science Council of Taiwan in Grant NSC 97-2628-B-415-001-MY3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Min-Guan Lin and Bo-En Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lin, MG., Chen, BE., Liang, WC. et al. Site-Saturation Mutagenesis of Leucine 134 of Bacillus licheniformis Nucleotide Exchange Factor GrpE Reveals the Importance of this Residue to the Co-chaperone Activity. Protein J 29, 365–372 (2010). https://doi.org/10.1007/s10930-010-9261-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9261-5