Abstract
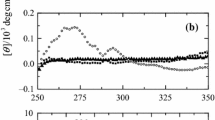
The structural stability of metmyoglobin in organic solvents and cosolvents was investigated aiming the choice of a suitable medium to perform its dissolution with maintenance of the native folding. The spectroscopic behavior of metmyoglobin solution in UV–Visible and circular dichroism was used to evaluate the solubility and the secondary structure. The results were dependable of the chemical structure of the organic compounds, their polarity and content, in the case of cosolvents. Protic solvents showed better ability than the aprotic ones for the biomolecule dissolution, since they are able to establish hydrogen bonds. Solvents with high polarity usually damage the secondary structure of the protein. Myoglobin was dissolved in pure methanol, ethylene glycol and glycerol. The secondary structure was retained in some extent. The controlled addition of sodium dodecyl sulfate to myoglobin aqueous solution changed the surface moiety of the protein. The complex was extracted to hexane with efficiency of 77%.









Similar content being viewed by others
Abbreviations
- CD:
-
Circular dichroism
- SDS:
-
Sodium dodecyl sulfate
- C50 :
-
Threshold content of the organic cosolvent in which myoglobin is able to retain its native folding
- PBS:
-
Phosphate buffer solution
- DMSO:
-
Dimethyl sulfoxide
- Metmb:
-
Metmyoglobin
References
Babu KR, Douglas DJ (2000) Biochemistry 39:14702–14710
Barteri M, Gaudiano MC, Santucci R (1996) BBA-Proteins Proteom 1295:51–58
Brantley RE, Smerdon SJ, Wilkinson AJ, Singleton EW, Olson JS (1993) J Biol Chem 268:6995–7010
Burova TV, Grinberg NV, Grinberg VY, Rariy RV, Klibanov AM (2000) BBA-Proteins Proteom 1478:309–317
Ferraz HC, Duarte LT, Di Luccio M, Alves TLM, Habert AC, Borges CP (2007) Braz J Chem Eng 24:101–118
Figueiredo KCS (2008) PhD thesis, Federal University of Rio de Janeiro, Rio de Janeiro, pp. 1–202
Glandieres JM, Calmettes P, Martel P, Zentz C, Massat A, Ramstein J, Alpert B (1995) Eur J Biochem 227:241–248
Guo YZ, Clark DS (2001) BBA-Proteins Proteom 1546:406–411
Herskovits T, Gadegbeku B, Jaillet H (1970) J Biol Chem 245:2588–2598
Jackson M, Mantsch HH (1991) BBA-Proteins Proteom 1078:231–235
Kaim W, Schwedersky B (1994) Bioinorganic chemistry: inorganic elements in the chemistry of life—an introduction and guide. Wiley, New York
Kelly SM, Jess TJ, Price NC (2005) BBA-Proteins Proteom 1751:119–139
Khmelnitsky YL, Mozhaev VV, Belova AB, Sergeeva MV, Martinek K (1991) Eur J Biochem 198:31–41
Klibanov AM (1997) Trends Biotechnol 15:97–100
Kony DB, Hunenberger PH, van Gunsteren WF (2007) Protein Sci 16:1101–1118
Li QC, Mabrouk PA (2003) J Biol Inorg Chem 8:83–94
Lim LY, Wan SC (1994) Drug Dev Ind Pharm 20:1007–1020
Manavalan P, Johnson WC Jr (1987) Anal Biochem 167:76–85
Matsuura J, Powers ME, Manning MC, Shefter E (1993) J Am Chem Soc 115:1261–1264
Mattos C, Ringe D (2001) Curr Opin Struc Biol 11:761–764
Meyer JD, Manning MC (1998) Pharm Res 15:188–193
Rosell CM, Vaidya AM, Halling PJ (1995) BBA-Proteins Proteom 1252:158–164
Shikama K (2006) Prog Biophys Mol Biol 91:83–162
Sreerama N, Woody RW (2000) Anal Biochem 287:252–260
Sugawara Y, Matsuoka A, Kaino A, Shikama K (1995) Biophys J 69:582–592
Tofani L, Feis A, Snoke RE, Berti D, Baglioni P, Smulevich G (2004) Biophys J 87:1186–1195
Acknowledgments
The authors would like to thank Capes and CNPQ for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Figueiredo, K.C.S., Ferraz, H.C., Borges, C.P. et al. Structural Stability of Myoglobin in Organic Media. Protein J 28, 224–232 (2009). https://doi.org/10.1007/s10930-009-9187-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-009-9187-y