Abstract
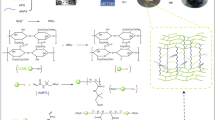
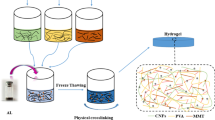
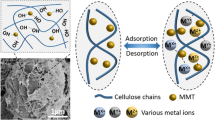
Carboxymethyl cellulose-based hydrogel enhanced by two-dimensional montmorillonite nanosheets (CMC-MMTs Gel) has been successfully synthesized throguh polymerization for methylene blue (MB) removal from water. Structure characterization shows that the introduction of two-dimensional montmorillonite nanosheets (2D MMTs) into CMC-based hyddrogel results in a roguh surface and increased porosity. Adsorption tests demonstrate that the adsorption rate and adsorption capacity of CMC-based hydrogel increase significantly with the increase in 2D MMTs content, and a high adsorption capacity of 410 mg g−1 can be reached. After 5 cycles, CMC-MMTs Gel still exhibits good removal performance indicating a good reusability and structural stability. The enhanced MB removal performance and improved structural stability might be due to 2D MMTs and the increased porosity. FTIR and XPS characterizations demonstrate that the adsorption mechanism of MB onto CMC-MMTs Gel might be attributed to electrostatic adsorption and ion-exchange between MB molecules and MMTs, and the interactions between MB molecules and the hydroxyl and carboxyl groups of CMC.









Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Karimifard S, Alavi Moghaddam MR (2018) Application of response surface methodology in physicochemical removal of dyes from wastewater: a critical review. Sci Total Environ 640–641:772–797. https://doi.org/10.1016/j.scitotenv.2018.05.355
Yadav VB, Gadi R, Kalra S (2019) Clay based nanocomposites for removal of heavy metals from water: a review. J Environ Manage 232:803–817. https://doi.org/10.1016/j.jenvman.2018.11.120
Han H, Rafiq MK, Zhou T et al (2019) A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J Hazard Mater 369:780–796. https://doi.org/10.1016/j.jhazmat.2019.02.003
Pandey S (2017) A comprehensive review on recent developments in bentonite-based materials used as adsorbents for wastewater treatment. J Mol Liq 241:1091–1113. https://doi.org/10.1016/j.molliq.2017.06.115
Foroutan R, Mohammadi R, Sohrabi N et al (2020) Calcined alluvium of agricultural streams as a recyclable and cleaning tool for cationic dye removal from aqueous media. Environ Technol Innov. https://doi.org/10.1016/j.eti.2019.100530
Foroutan R, Mohammadi R, Razeghi J, Ramavandi B (2019) Performance of algal activated carbon/Fe3O4 magnetic composite for cationic dyes removal from aqueous solutions. Algal Res. https://doi.org/10.1016/j.algal.2019.101509
Kang S, Zhao Y, Wang W et al (2018) Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl Surf Sci 448:203–211. https://doi.org/10.1016/j.apsusc.2018.04.037
Thakur S, Pandey S, Arotiba OA (2016) Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr Polym 153:34–46. https://doi.org/10.1016/j.carbpol.2016.06.104
Liu C, Omer AM, Ouyang XK (2018) Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: Isotherm and kinetic studies. Int J Biol Macromol 106:823–833. https://doi.org/10.1016/j.ijbiomac.2017.08.084
Astuti W, Sulistyaningsih T, Kusumastuti E et al (2019) Thermal conversion of pineapple crown leaf waste to magnetized activated carbon for dye removal. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.121426
Zhao Y, Kang S, Qin L et al (2020) Self-assembled gels of Fe-chitosan/montmorillonite nanosheets: dye degradation by the synergistic effect of adsorption and photo-Fenton reaction. Chem Eng J. https://doi.org/10.1016/j.cej.2019.122322
Li X, Zhao X, Zhou X, Yang B (2021) Phosphate recovery from aqueous solution via struvite crystallization based on electrochemical-decomposition of nature magnesite. J Clean Prod. https://doi.org/10.1016/j.jclepro.2021.126039
Zhu Y, Fan W, Zhou T, Li X (2019) Removal of chelated heavy metals from aqueous solution: a review of current methods and mechanisms. Sci Total Environ 678:253–266. https://doi.org/10.1016/j.scitotenv.2019.04.416
Peng W, Li H, Liu Y, Song S (2017) A review on heavy metal ions adsorption from water by graphene oxide and its composites. J Mol Liq 230:496–504. https://doi.org/10.1016/j.molliq.2017.01.064
Aichour A, Zaghouane-Boudiaf H, Iborra CV, Polo MS (2018) Bioadsorbent beads prepared from activated biomass/alginate for enhanced removal of cationic dye from water medium: Kinetics, equilibrium and thermodynamic studies. J Mol Liq 256:533–540. https://doi.org/10.1016/j.molliq.2018.02.073
Sharma S, Hasan A, Kumar N, Pandey LM (2018) Removal of methylene blue dye from aqueous solution using immobilized Agrobacterium fabrum biomass along with iron oxide nanoparticles as biosorbent. Environ Sci Pollut Res 25:21605–21615. https://doi.org/10.1007/s11356-018-2280-z
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462. https://doi.org/10.1016/j.cej.2016.09.029
Foroutan R, Zareipour R, Mohammadi R (2018) Fast adsorption of chromium (VI) ions from synthetic sewage using bentonite and bentonite/bio-coal composite: a comparative study. Mater Res Express. https://doi.org/10.1088/2053-1591/aaebb9
Foroutan R, Khoo FS, Ramavandi B, Abbasi S (2017) Heavy metals removal from synthetic and shipyard wastewater using phoenix dactylifera activated carbon. Desalin Water Treat 82:146–156. https://doi.org/10.5004/dwt.2017.20908
Khan M, Lo IMC (2016) A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res 106:259–271. https://doi.org/10.1016/j.watres.2016.10.008
Hong TT, Okabe H, Hidaka Y, Hara K (2018) Radiation synthesis and characterization of super-absorbing hydrogel from natural polymers and vinyl monomer. Environ Pollut 242:1458–1466. https://doi.org/10.1016/j.envpol.2018.07.129
Salahuddin NA, EL-Daly HA, El Sharkawy RG, Nasr BT, (2018) Synthesis and efficacy of PPy/CS/GO nanocomposites for adsorption of ponceau 4R dye. Polymer (Guildf) 146:291–303. https://doi.org/10.1016/j.polymer.2018.04.053
Bozoğlan BK, Duman O, Tunç S (2020) Preparation and characterization of thermosensitive chitosan/carboxymethylcellulose/scleroglucan nanocomposite hydrogels. Int J Biol Macromol 162:781–797. https://doi.org/10.1016/j.ijbiomac.2020.06.087
Kancı Bozoğlan B, Duman O, Tunç S (2021) Smart antifungal thermosensitive chitosan/carboxymethylcellulose/scleroglucan/montmorillonite nanocomposite hydrogels for onychomycosis treatment. Colloids Surfaces A. https://doi.org/10.1016/j.colsurfa.2020.125600
Foroutan R, Ahmadlouydarab M, Ramavandi B, Mohammadi R (2018) Studying the physicochemical characteristics and metals adsorptive behavior of CMC-g-HAp/Fe3O4 nanobiocomposite. J Environ Chem Eng 6:6049–6058. https://doi.org/10.1016/j.jece.2018.09.030
Hong TT, Okabe H, Hidaka Y et al (2019) Radiation induced modified CMC-based hydrogel with enhanced reusability for heavy metal ions adsorption. Polymer (Guildf). https://doi.org/10.1016/j.polymer.2019.121772
Salama A, Shukry N, El-Sakhawy M (2015) Carboxymethyl cellulose-g-poly(2-(dimethylamino) ethyl methacrylate) hydrogel as adsorbent for dye removal. Int J Biol Macromol 73:72–75. https://doi.org/10.1016/j.ijbiomac.2014.11.002
Pereira AGB, Rodrigues FHA, Paulino AT et al (2021) Recent advances on composite hydrogels designed for the remediation of dye-contaminated water and wastewater: a review. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.124703
Zheng Y, Wang A (2010) Enhanced adsorption of ammonium using hydrogel composites based on chitosan and halloysite. J Macromol Sci Part A 47:33–38. https://doi.org/10.1080/10601320903394421
Chiew CSC, Poh PE, Pasbakhsh P et al (2014) Physicochemical characterization of halloysite/alginate bionanocomposite hydrogel. Appl Clay Sci 101:444–454. https://doi.org/10.1016/j.clay.2014.09.007
Vahidhabanu S, Karuppasamy D, Adeogun AI, Babu BR (2017) Impregnation of zinc oxide modified clay over alginate beads: A novel material for the effective removal of congo red from wastewater. RSC Adv 7:5669–5678. https://doi.org/10.1039/c6ra26273b
Shirsath SR, Patil AP, Patil R et al (2013) Removal of Brilliant Green from wastewater using conventional and ultrasonically prepared poly(acrylic acid) hydrogel loaded with kaolin clay: a comparative study. Ultrason Sonochem 20:914–923. https://doi.org/10.1016/j.ultsonch.2012.11.010
Rahman MM, Rimu SH, Biswas S et al (2020) Preparation of poly(acrylic acid) exfoliated clay composite by in-situ polymerisation for decolouration of methylene blue from wastewater. Int J Environ Anal Chem 00:1–17. https://doi.org/10.1080/03067319.2020.1813732
Yi JZ, Zhang LM (2007) Studies of sodium humate/polyacrylamide/clay hybrid hydrogels. I. Swelling and rheological properties of hydrogels. Eur Polym J 43:3215–3221. https://doi.org/10.1016/j.eurpolymj.2007.05.023
Chen T, Yuan Y, Zhao Y et al (2019) Preparation of montmorillonite nanosheets through freezing/thawing and ultrasonic exfoliation. Langmuir. https://doi.org/10.1021/acs.langmuir.8b04171
Wang W, Zhao Y, Yi H et al (2018) Preparation and characterization of self-assembly hydrogels with exfoliated montmorillonite nanosheets and chitosan. Nanotechnology. https://doi.org/10.1088/1361-6528/aa9ba4
Yi H, Ai Z, Zhao Y et al (2020) Design of 3D-network montmorillonite nanosheet/stearic acid shape-stabilized phase change materials for solar energy storage. Sol Energy Mater Sol Cells. https://doi.org/10.1016/j.solmat.2019.110233
Qin L, Zhao Y, Wang L et al (2020) Preparation of ion-imprinted montmorillonite nanosheets/chitosan gel beads for selective recovery of Cu(II) from wastewater. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.126560
Wang W, Wang J, Zhao Y et al (2020) High-performance two-dimensional montmorillonite supported-poly(acrylamide-co-acrylic acid) hydrogel for dye removal. Environ Pollut. https://doi.org/10.1016/j.envpol.2019.113574
Chen L, Zhao Y, Chen T et al (2019) Correlation of aspect ratio of montmorillonite nanosheets with the colloidal properties in aqueous solutions. Results Phys. https://doi.org/10.1016/j.rinp.2019.102526
Temuujin J, Jadambaa T, Burmaa G et al (2004) Characterisation of acid activated montmorillonite clay from Tuulant (Mongolia). Ceram Int 30:251–255. https://doi.org/10.1016/S0272-8842(03)00096-8
Zhang J, Jia G, Wanbin Z et al (2021) Nanoencapsulation of zeaxanthin extracted from Lycium barbarum L. by complex coacervation with gelatin and CMC. Food Hydrocoll 112:106280. https://doi.org/10.1016/j.foodhyd.2020.106280
Reddy BV, Rao GR (2008) Vibrational spectra and modified valence force field for N, N’-methylenebisacrylamide. Indian J Pure Appl Phys 46:611–616
Soares VLP, Nascimento RSV, Menezes VJ, Batista L (2004) TG characterization of organically modified montmorillonite. J Therm Anal Calorim 75:671–676. https://doi.org/10.1023/B:JTAN.0000027161.10803.60
Lv G, Li Z, Jiang WT et al (2015) Interlayer configuration of ionic liquids in a Ca-montmorillonite as evidenced by FTIR, TG-DTG, and XRD analyses. Mater Chem Phys 162:417–424. https://doi.org/10.1016/j.matchemphys.2015.06.008
Jeong D, Kim C, Kim Y, Jung S (2020) Dual crosslinked carboxymethyl cellulose/polyacrylamide interpenetrating hydrogels with highly enhanced mechanical strength and superabsorbent properties. Eur Polym J. https://doi.org/10.1016/j.eurpolymj.2020.109586
Peighambardoust SJ, Aghamohammadi-Bavil O, Foroutan R, Arsalani N (2020) Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int J Biol Macromol 159:1122–1131. https://doi.org/10.1016/j.ijbiomac.2020.05.093
Kumararaja P, Manjaiah KM, Datta SC et al (2018) Chitosan-g-poly(acrylic acid)-bentonite composite: a potential immobilizing agent of heavy metals in soil. Cellulose 25:3985–3999. https://doi.org/10.1007/s10570-018-1828-x
Ji J, Zeng C, Ke Y, Pei Y (2017) Preparation of poly(acrylamide-co-acrylic acid)/silica nanocomposite microspheres and their performance as a plugging material for deep profile control. J Appl Polym Sci 134:1–11. https://doi.org/10.1002/app.45502
Rakhsh F, Golchin A, Beheshti Al Agha A, Alamdari P (2017) Effects of exchangeable cations, mineralogy and clay content on the mineralization of plant residue carbon. Geoderma 307:150–158. https://doi.org/10.1016/j.geoderma.2017.07.010
He Y, Bin JD, Chen J et al (2018) Synthesis of MnO2 nanosheets on montmorillonite for oxidative degradation and adsorption of methylene blue. J Colloid Interface Sci 510:207–220. https://doi.org/10.1016/j.jcis.2017.09.066
Chen Y, Chen L, Bai H, Li L (2013) Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J Mater Chem A 1:1992–2001. https://doi.org/10.1039/c2ta00406b
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. https://doi.org/10.1016/j.cej.2009.09.013
Duman O, Tunç S, Polat TG, Bozoǧlan BKI (2016) Synthesis of magnetic oxidized multiwalled carbon nanotube-κ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr Polym 147:79–88. https://doi.org/10.1016/j.carbpol.2016.03.099
Duman O, Polat TG, Diker CÖ, Tunç S (2020) Agar/κ-carrageenan composite hydrogel adsorbent for the removal of Methylene Blue from water. Int J Biol Macromol 160:823–835. https://doi.org/10.1016/j.ijbiomac.2020.05.191
Wang W, Ni J, Chen L et al (2020) Synthesis of carboxymethyl cellulose-chitosan-montmorillonite nanosheets composite hydrogel for dye effluent remediation. Int J Biol Macromol 165:1–10. https://doi.org/10.1016/j.ijbiomac.2020.09.154
Gan W, Shang X, Li XH et al (2019) Achieving high adsorption capacity and ultrafast removal of methylene blue and Pb2+ by graphene-like TiO2@C. Colloids Surfaces A 561:218–225. https://doi.org/10.1016/j.colsurfa.2018.10.079
Ovchinnikov OV, Evtukhova AV, Kondratenko TS et al (2016) Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib Spectrosc 86:181–189. https://doi.org/10.1016/j.vibspec.2016.06.016
Pang J, Fu F, Ding Z et al (2017) Adsorption behaviors of methylene blue from aqueous solution on mesoporous birnessite. J Taiwan Inst Chem Eng 77:168–176. https://doi.org/10.1016/j.jtice.2017.04.041
Wang W, Bai H, Zhao Y et al (2019) Synthesis of chitosan cross-linked 3D network-structured hydrogel for methylene blue removal. Int J Biol Macromol 141:98–107. https://doi.org/10.1016/j.ijbiomac.2019.08.225
Yang JY, Jiang XY, Jiao FP, Yu JG (2018) The oxygen-rich pentaerythritol modified multi-walled carbon nanotube as an efficient adsorbent for aqueous removal of alizarin yellow R and alizarin red S. Appl Surf Sci 436:198–206. https://doi.org/10.1016/j.apsusc.2017.12.029
Acknowledgements
The financial supports from the “Fundamental Research Funds for the Central Universities (WUT: 2020IVA082) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
QM characterization of samples, investigation and writing original draft; WW syntheses, investigation and data analysis; WG conceptualization, review and editing; LX review and editing; HL investigation; Shaoxian Song: resources, conceptualization and supervision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, Q., Wang, W., Ge, W. et al. Preparation of Carboxymethyl Cellulose-Based Hydrogel Supported by Two-Dimensional Montmorillonite Nanosheets for Methylene Blue Removal. J Polym Environ 29, 3918–3931 (2021). https://doi.org/10.1007/s10924-021-02166-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02166-7